Chemical Reactions
advertisement

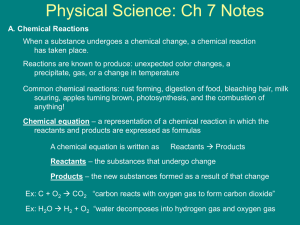
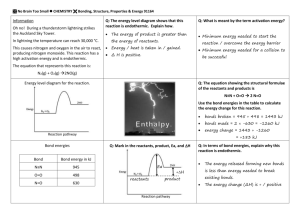
7.1 The Nature of Chemical Reactions Chemical Reactions - a reaction in which one or more substances are converted into different new substances with different properties than originally started with. Chemical reactions break chemical bonds and rearrange atoms. The reactants are the starting materials in a chemical reaction. The products are the substances that are formed by a chemical reaction. o Signs of a chemical reaction o release of gas or bubbles – CO2 o formation of a solid - precipitate o color change o energy released as light or heat – combustion Rx ENERGY AND REACTIONS o All chemical reactions always involve changes in energy between the reactants and products. o Energy must be added to break bonds. Activation Energy o Energy added as heat, electricity, sound, or light Energy is always conserved in a chemical reaction. o Law of Conservation of Energy – Energy can not be created nor destroyed in a chemical reaction. o Chemical energy - the energy stored in the bonds of compounds o The total energy before the reaction is equal to the total energy of the products and their surroundings. Reactions that release energy are exothermic o Some reactions release energy to their surroundings and are called exothermic. Ex. Combustion reactions give off intense heat and light. o More energy is released from the reactants than stored in the bonds of the new product. Reactions that absorb energy are endothermic o Some reactions need to absorb heat from their surroundings to proceed. These reactions are called endothermic. o Because the reaction is endothermic, it absorbs heat from the surrounding environment, reducing the temperature. o More energy is stored in the bonds of the products than bonds of the reactants. o An example of an endothermic reaction is that which takes place inside of an instant 'cold pack'. Commercial cold packs usually consist of 2 compounds - urea and ammonium chloride in separate containers within a plastic bag. When the bag is bent and the inside containers are broken, the two compounds mix together and begin to react. Because the reaction is endothermic, it absorbs heat from the surrounding environment and the bag gets cold. 7.3 Types of Chemical Reactions Classifying Reactions You can use patterns in chemical reactions to identify kinds of reactions and predict the products of the chemical reaction Synthesis reactions combine substances o A synthesis reaction is a chemical change in which two or more substances combine to form a new more complex compound. o General Form A + B ---> AB o Photosynthesis – the production of glucose and oxygen from sunlight, carbon dioxide and water o Decomposition reactions break substances apart o A decomposition reaction is a chemical change in which a single compound breaks down to form two or more simpler substances. o A decomposition reaction is the opposite of a synthesis reaction o General form AB ---> A + B o Electrolysis of water - passing an electric current through water causes the water to break down into the elements, hydrogen and oxygen. 2 H2O ---> 2 H2 + O2 Electrolysis o Combustion Reaction o A combustion reaction is the oxidation of an organic compound, in which heat is released. o Combustion reactions use oxygen as a reactant o These are exothermic, and form water and carbon dioxide as products o The burning of methane: o Single-displacement reactions, elements trade places o When one element trades places with another element in a compound. These reactions come in the general form of: o General form A + BC ---> AC + B o Potassium replaces hydrogen in water to make potassium hydroxide and hydrogen gas: 2K + 2H(OH) ---> 2K(OH) + H2 o Double-displacement reactions, ions appear to be exchanged between compounds o the anions and cations of two different molecules switch places, forming two entirely different compounds. o General form: AB + CD ---> AD + CB o Reaction of lead (II) nitrate with potassium iodide to form lead (II) iodide and potassium nitrate: Pb(NO3)2 + 2 KI ---> PbI2 + 2 KNO3 o Electrons and Chemical Reactions o Radicals have electrons available for bonding o fragments of molecules that have at least one electron available for bonding (forms covalent bonds) o Reduction/oxidation (Redox) reaction: a reaction that occurs when electrons are transferred from one reactant to another o Metals are Oxidized (lose electrons) o Non-metals are Reduced (gain electrons) 7.2 Chemical Equations Describing Reactions o Chemical Equations uses symbols to represent a chemical reaction and show the relationship between the reactants and the products. o Follows The Law Conservation of Mass o Balanced equations show Mole ratios o Mole ratios can be turned into masses Balancing Chemical Equation 1. The is read as “yields” or “produces.” 2. When the number of atoms on the right side of the equation matches the number of atoms on the left side of the equation, the equation is said to be balanced. 3. 4. When balancing reactions, keep your hands off the subscripts! Use only coefficients to balance chemical equations. 5. Example 6. Write the balanced equation for the reaction between chlorine and sodium bromide, which produces bromine and sodium chloride. 8. Explanation 9. First write the chemical formulas—be on the lookout for the diatomic elements (such as Cl2): 10.Cl2 + NaBr Br2 + NaCl 11.Next, find the reagent with the scariest subscripts. In this case, start with Cl2. You need a coefficient of 2 in front of NaCl, which then requires a coefficient of 2 in front of NaBr. The balanced equation becomes 12.Cl2 + 2NaBr Br2 + 2NaCl 13.Finally, count up everything to make sure you balanced the equation correctly. You have two chlorine atoms, two sodium atoms, and two bromines on the reactants side and two bromines, two sodiums, and two chlorines on the products side. You’re done. 14.Example 15.Write the balanced equation for the reaction between aluminum sulfate and calcium chloride, which produces aluminum chloride and calcium sulfate. 16.Explanation 17.Write the chemical formulas on their correct sides: 18.Al2(SO4)3 + CaCl2 AlCl3 + CaSO4 19.In this reaction, the aluminum sulfate looks the most complicated, so start there. Look at what happens with sulfate—since it remains sulfate on the right side of the reaction, treat it as a unit. You have three on the left side and only one on the right side, so place a coefficient of 3 in front of calcium sulfate. Now deal with the aluminum. You have three on the left and one on the right, so place a coefficient of 2 in front of aluminum chloride. Last, you must place a coefficient of 3 in front of calcium chloride. 20.Al2(SO4)3 + 3CaCl2 2AlCl3 + 3CaSO4 21.Count the atoms on both sides of the reaction and you’ll see that you’re done. 7.4 Reactions Rates and Equilibrium Anything that increases contact between particles will increase the rate of reactions. o Reactions may be very slow (years) or quick (combustion) o Most go faster at higher temperature (kinetic theory) o Massive bulky items react more slowly o Large surface area speeds up reactions ( crushed vs sugar cube) o Higher concentrations increase reaction rates. (cleaners) o Reactions are faster under higher pressure (cookers) Cataylst o Facilitate, usually speeding up a reaction but is not changed by the reaction (can be reused) o Inhibitors prevent or slow down a catalyst from working o Enzymes are biological catalysts. o A substrate is the reactant that the catalyzed, or changed, by the enzyme. o Location on the enzyme where the substrate fits is called the active site. 1.