PRESBYTERIAN HOMES, INC.
advertisement

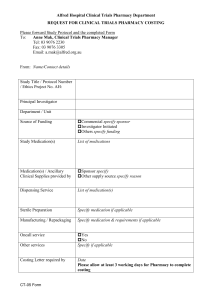
POLICY RESEARCH/INVESTIGATION/CLINICAL TRIALS POLICY: The Administrator must approve any research project, investigational study or clinical trial. Research is defined to be a systematic investigation designed to develop or contribute to generalized knowledge. This includes registries, retrospective, observational or prospective clinical studies, surveys and laboratory studies. OBJECTIVE: Resident rights will be maintained at all times as they relate to participation in research, investigational or clinical trials. These rights include informed consent, confidentiality, and consequence of knowledge. PROCEDURE: 1. The Administrator, in consultation with the DON and Medical Director will make determination as to whether a research activity may be allowed at the facility. A written protocol of the proposed activity will be available during the decision making process. 2. Determination will include if the facility staff is competent to provide the care or treatment outlined in the protocol. 3. The Administrator will assure that the activity does not violate the organizational Code of Ethics. 4. The Ethics Committee will be consulted in the event that this represents an ethical dilemma. 5. Once approved, the principal investigator must present the following information to the Administrator in writing before beginning the activity: A. Notification of residents concerning the protocol, benefits, risks, potential outcomes and consequences as well as alternative courses of intervention. B. Signed informed consent of residents involved which is explained by the primary investigator with a copy maintained in the resident’s medical record. C. A statement related to resident maintenance of confidentiality. 6. Physician orders for care and a copy of any protocol shall be maintained on the medical record. 7. When the investigation protocol or clinical trial involves medication administration, the facility will involve the pharmacist in the discussion as well as in the long term monitoring of the resident’s outcomes. 88 8. The pharmacy will maintain responsibility for procurement and inventory control of the medication in the pharmacy, while the medication delivery system shall dictate facility inventory control. (Ex. Unit dose vs. Bubble pack). 9. The pharmacy will additionally be responsible for returning unused portions of the medication to the primary investigator at the completion of the study. 10. Nurses participating in the clinical trial shall meet the following criteria: A. Must be permitted by state law to administer the medication by the prescribed route and have been authorized to do so by the principal investigator B. Shall complete an orientation concerning the medication, administration guidelines, the pharmacological action and use as well as the pharmacological monitoring parameters according to the approved protocol 11. The Administrator, participating residents/subjects or their family shall be given a copy of the final report of the outcomes. shall be given progress reports of the activity as well as a final report of the outcomes. 12. The facility will maintain a research directory of ongoing research projects at the facility. 13. Approval to publish results of research activities must be granted by the Board of Directors. Revised---March 2001 89