Antacid Analysis
advertisement

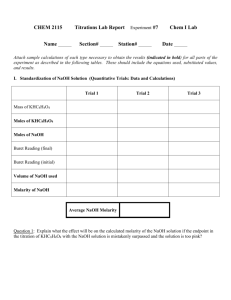
Chemistry 11 Santa Monica College Evaluating the Cost-Effectiveness of Antacids Objectives In this exercise, two commercially available antacids are evaluated and compared by: determining the number of moles of H+ neutralized per gram of each antacid. calculating the cost effectiveness of each antacid. Background Acid indigestion is a common ailment caused by the overproduction of stomach acid, HCl. Overthe-counter antacids provide some relief from the symptoms of acid indigestion. They are generally made up of some mixture of weak bases such as Mg(OH)2, Al(OH)3, and CaCO3 that can react with HCl as shown in these net ionic equations: H+ (aq) + OH- (aq) H2O (l) 2H+ (aq) + CO3 2- (aq) H2O (l) + CO2 (g) In this exercise, the method of titration will be used to determine the number of moles of H+ neutralized per gram of antacid. In the “back-titration,” a portion of antacid will be mixed with an excess of HCl. The H+ that has not reacted with the antacid is then titrated with standardized NaOH in the presence of the indicator bromophenol blue to a blue end point. The end point, is defined as the volume of OH- needed to see a color change. Because only the tiniest excess of OH- over H+ can cause the color change of an indicator, the end point is a close approximation of the equivalence point. (The difference between the end point and the equivalence point is known as the titration error.) At the equivalence point, the number of moles of OH- added is equal to the number of moles of excess H+ that had not been neutralized by the antacid. By knowing the total moles of HCl added, one can then calculate the number of moles of H+ neutralized by the antacid. Total moles of H+ = moles of H+ neutralized by antacid + moles of H+ neutralized by NaOH Because the antacid includes both OH- and CO3 2-, it is not possible to calculate the number of moles of each of these ion species independently. Instead, the number of H+ neutralized by the antacid is found. The amount of antacid required to neutralize one mole of H+ neutralized is said to be one “equivalent.” total equivalent of antacid = total mole of H+ neutralized The more cost effective antacid is the one that costs fewer dollars per equivalent. Evaluating the Cost Effectiveness of Antacids Page 1 of 3 Chemistry 11 Santa Monica College Materials and Equipment Antacid tablets – Rolaids ®, Tums ®, etc. It is preferable to use tablets that are white and have no added flavor. Mortar and pestle Two Ehrlenmeyer flasks and at least two 100 mL beakers Bunsen burner Ring stand with ring clamp and wire gauze Standardized HCl solution – about 0.1M Standardized NaOH solution – about 0.1M Bromophenol blue indicator solution Two Burets* Safety Wear goggles at all times during this experiment. Take care in handling both HCl and NaOH. Should either of these solutions come in contact with skin or eyes, rinse thoroughly with water. Allow enough time for the boiled antacid solution to come to room temperature before touching it. Procedure A. Preparation of Antacid Sample 1. Choose an antacid and record its name on the data sheet. With a mortar and pestle, crush one tablet of antacid to as fine a powder as possible. 2. Weigh out between 0.3 – 0.4 g of the powdered antacid into a pre-weighed Erlenmeyer flask (250 mL). Record the mass of the antacid sample to 0.001g. B. Preparation of Burets 1. Obtain two burets from the stockroom and, with soap and water, wash them with a brush. Rinse them well with deionized water (When the buret is cleaned properly, there should be no water droplets clinging to the inside of the buret.) One buret will be filled with the standardized HCl and the other with standardized NaOH. Label them accordingly and record their concentrations as indicated on the label. 2. It is important that the concentration of the acid and base are not diluted with any of the water left over from cleaning. So, before filling the burets, rinse them well with at least two 4-mL portions of either HCl or NaOH as appropriate. 3. Using a beaker, fill the burets with HCl and NaOH. Note that bubbles take up space, so make sure to dislodge any bubbles that might be stuck in the tip of the buret by lightly tapping the tip or by letting a portion of the solution run rapidly through the tip. Record the initial buret readings for both HCl and NaOH. Evaluating the Cost Effectiveness of Antacids Page 2 of 3 Chemistry 11 Santa Monica College C. Addition of excess HCl to the antacid 1. Record the initial volume of HCl in the buret and add approximately 40 mL of standardized HCl to the prepared antacid sample. Record the final volume of HCl in the buret to 0.01 mL. 2. In order to dissolve as much as of the antacid as possible and to drive off as much dissolved CO2 as possible, gently boil the mixture of antacid + HCl for about two minutes. Cool the mixture to room temperature. There may be a significant amount of substances that are not dissolved in your mixture. Because the active ingredients of an antacid are quite water soluble, the solids will not affect the results. The solids are a mixture of inactive ingredients such as the coating and some binding compounds. 3. Add 6 - 8 drops of bromophenol blue indicator solution. The solution should be yellow if there is excess H+ in solution. If the solution is green, blue, or a mixture of both green and blue, there is excess OH- in solution and more HCl must be added. From the buret, add about 20 ml of HCl, again recording to 0.01 mL. Make sure there is enough HCl in the buret. Remember, it is not possible to measure the volume of HCl if the level drops below the 50.00 mL mark! Assess the color of the solution. If it is not yellow, repeat the addition of HCl until it is. D. Titration of excess HCl 1. Begin the neutralization of the excess HCl with the addition of standardized NaOH. Record the initial volume on the NaOH buret to 0.01 mL. Begin the titration by slowly adding the NaOH in 1 mL increments. Mix well between additions by swirling the flask. As more and more NaOH is added, the solution will turn from yellow to green and then finally blue. The titration reaches the end point when the addition of one to three drops of NaOH turn the solution blue and that color is stable with mixing for at least 20 seconds. Record the final buret reading and calculate the number of OH- moles added. E. Back-Titration --- Only necessary if too much NaOH has been added and the end point has been exceeded 1. If your solution is intensely blue – you’ve added too much NaOH! Don’t panic, this error can be corrected. Add enough of the standardized HCl to make the solution yellow. (About 5 - 8 mL) Record the new total volume of HCl. Again from the buret, add NaOH slowly until the solution turns blue. F. Repeat the procedure with another brand of antacid. Make every effort to reach an endpoint that has equivalent in its “blueness.” Clean – Up 1. Before returning burets to the stockroom, rinse them thoroughly with deionized water. 2. Wash glassware with soap and water and rinse thoroughly with deionized water. Evaluating the Cost Effectiveness of Antacids Page 3 of 3