The Synthesis and Analysis of a Nitrite Complex
advertisement

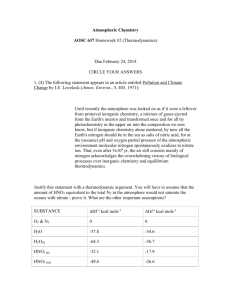
The Synthesis and Analysis of a Nitrite Complex Introduction Many chemists are in the business of making target molecules which they hope will have beneficial chemical or physical properties. This experiment will require a synthesis of one of a group of compounds with the general formula K2MM(NO2)6 where M is an alkaline earth metal ion and M is a transition metal ion with a charge of +2. Once a chemist has synthesized a compound, the next step is the characterization of the compound. Characterization involves proving that the target molecule has indeed been synthesized, and usually uses sophisticated instrumentation to positively identify the structure of molecule. Once you have synthesized the nitrite complex, you will analyze it using a gasometric technique. Some background about these compounds will now be discussed briefly. The complex ion in the above compound is M(NO2)64-. There are 6 nitrite ligands surrounding a central transition metal ion. A ligand is a molecule or ion that surrounds a central metal ion. The geometry of this ion is what you expect when placing 6 species around a center atom (recall VSEPR!). A ligand has lone pairs of electrons and (as we will find out later) is therefore a Lewis base. The metal atom has empty available bonding orbitals which accepts these electron pairs of the ligand, and is therefore a Lewis acid. The potassium and alkaline metal earth ions are held to the complex ion by electrostatic attractive forces. If we consider all of the possible combination of Group 2 metals with all of the transition metals, there would be a large number of different possible products that could be made. We will limit these possibilities based on solubility. The following combinations are recommended because of their low solubility in water, and are therefore easily separated by filtration: Table I. Recommended Combinations of M with M If M is Ni2+ Co2+ Cu2+ Choose one of the following for M Ca2+, Sr2+, or Ba2+ Sr2+, or Ba2+ Sr2+ Safety: 1. KNO2 is toxic if ingested, as well as a strong oxidizing agent and should be treated with care. Dispose of any excess in the container provided. 2. The salts of cobalt(II), nickel(II) and strontium and barium are toxic. Dispose of any remaining product in the waste container provided. 3. Acetone is flammable. There should be no flames present when using acetone. 1 Week 1 - Synthesis You will either be assigned M and M or be allowed to choose your own according to Table I. Weigh out about 0.01 mole of the group 2 metal chloride and 0.01 mole of the transition metal chloride. Record the actual masses in the notebook. Dissolve both of these in a minimum amount of pure water (about 10 mL) in the same beaker. You may need to heat the solution (on low) to dissolve these salts. In another beaker, weigh out 0.1 mole of potassium nitrite and dissolve it in about 10 mL of water. Add this slowly to the beaker with the M and M chloride salts, stirring as you pour the solutions together. Prepare an ice bath, and put the beaker with the mixture in the ice bath. While the solid in the beaker is settling, set up a filtration apparatus (Buchner funnel) with an aspirator. Decant the liquid layer into the appropriate waste container. Add a 5 mL portion of cold pure water to which has been added a small amount (about the size of 1 grain of rice) of potassium nitrite. Stir this well and transfer to the Buchner funnel in which filter paper has been placed. Turn on the water to the aspirator. Add a 5 mL portion of acetone to the Buchner funnel. Repeat this 2 more times. Put the filter paper with product in a drying oven at about 60 ° C. While your product is drying, label and weigh an empty, dry bottle with a lid, and record its mass. When your product is dry (no acetone smell remains) transfer the product to the weighed bottle and weigh again, recording the total mass. Put the bottle back into the oven and heat again for another 1015 minutes. Allow the bottle to cool. Reweigh the bottle again. If there is little change from the first time, the product has been sufficiently dried. When you have dried your product, put the lid on the bottle and store it for the next week. Post-Lab Calculations 1. Calculate the theoretical yield. 2. Calculate the percent yield of your product and report this number to your instructor. Pre-lab Exercise – Synthesis of a Nitrite Complex 1. Write out balanced chemical reactions for the synthesis. Follow the symbolism used above, that is M is a group 2 metal, and M is the transition metal. Assume that the chloride ion is not a ligand, but is merely a spectator ion. 2. Define the following terms: Ligand Lewis base Lewis acid 3. What is the usual trend regarding the solubility of solids in solvents as the temperature changes? 4. Calculate the mass of the potassium nitrite to be used in this experiment. 5. Suppose a student needs to use CaCl2 in this synthesis. This generally comes as the dihydrate (CaCl22H2O). How much does the student need to weigh out to make sure that 0.010 mole of calcium ions are available? 2 The Synthesis and Analysis of a Nitrite Complex (continued) Week 2 - Analysis This analysis will give you evidence whether or not the formula of the compound you prepared previously is K2MM(NO2)6. When the nitrite complex is mixed with acid, it will react and produce nitrous acid: M(NO2)64- (aq) + 6 H+ (aq) --> 6HNO2 (aq) + M2+ (aq) Nitrous acid reacts quantitatively with the sulfamate ion according to HNO2 (aq) + NH2SO3- (aq) -- > N2 (g) + HSO4- (aq) + H2O (l) By measuring the volume of gas collected, the barometric pressure and the temperature, the total number of moles of dry nitrogen can be calculated using the ideal gas law. Recall that the total gas pressure must be corrected for the portion of water vapor in the collected gas sample using Dalton’s Law of partial pressures, and that any difference in water levels between the leveling bulb and the gas should also be corrected for. Both of these corrections are included in the following equation: PN2 = Patm - Pwater - h/13.6 where Pwater is the vapor pressure of water (see Table 2 at the end) at the measured temperature, Patm is the measured barometric pressure and h/13.6 is the correction for the difference in water levels. (The 13.6 is the units change from a pressure unit of mm water to mm Hg.) Once the moles of dry nitrogen gas are obtained, it becomes a matter of stoichiometry to find the mass percent nitrogen in the reacted sample. This can be compared to the mass percent nitrogen calculated from our assumed formula K2MM(NO2)6. Safety: The salts of cobalt(II), nickel(II) and strontium and barium are toxic. Dispose of any remaining product in the waste container provided. Procedure Use the presumed chemical formula K2MM(NO2)6 (with the appropriate molar mass) to calculate the mass of compound needed to produce 30 mL of gas. Accurately weigh out this amount (by difference) and put it into a small vial (1/2 dram size). Dissolve about 0.5 g of sulfamic acid in about 20 mL of water in a small beaker. Transfer this solution to the test tube (the reaction vessel) of the gas apparatus. (A written description of this apparatus is given in the appendix.) Carefully slide the small 3 vial into the reaction vessel, being careful to keep the vial upright so that the K2MM(NO2)6 does not mix with the sulfamic acid solution prematurely. Connect the gas collection apparatus as shown in Figure 1. When everything is assembled and ready, tip over the reaction vessel so that the sulfamic acid flows into the small vial. If the reaction begins before gas apparatus is completely assembled, or before an initial gas volume is measured, you must start over. The reaction should begin immediately. To make sure that the reaction has gone to completion, you should wait a minimum of 12 minutes before measuring and recording the final volume of gas, h, the temperature and the barometric pressure. Note that in most cases, h can be made to be zero by adjusting the height of the leveling bulb. Repeat the analysis until you have what you believe to be 3 successful trials. If too much gas is generated so the volume exceeds the measuring scale, the trial will need to be discarded. Figure 1 4 Post-Lab Calculations Calculate the mass percent nitrogen for each trial and average the results. Compute the theoretical percent nitrogen and compare this with your experimental results. Report the average on the summary report. Summary Report Trial 1 Trial 2 Trial 3 Trial 4 Mass of bottle (after weighing) _____ _____ _____ _____ Mass of bottle (before weighing) _____ _____ _____ _____ Mass of sample vial reacted _____ _____ _____ _____ Final buret reading _____ _____ _____ _____ Initial buret reading _____ _____ _____ _____ Volume of gas generated _____ _____ _____ _____ Barometric Pressure _____ _____ _____ _____ Room Temperature _____ _____ _____ _____ Vapor Pressure of Water (from Table 1) _____ _____ _____ _____ Moles of Nitrogen Collected _____ _____ _____ _____ Mass of Nitrogen in Sample _____ _____ _____ _____ Percent Nitrogen in Sample _____ _____ _____ _____ Average Percent Nitrogen _____ Theoretical Percent Nitrogen _____ 5 Table 2: Vapor Pressure of water at various temperatures (Data from CRC Handbook) Temp, oC 0.0 0.2 0.4 0.6 0.8 18 15.5 15.7 15.9 16.1 16.3 19 16.5 16.7 16.9 17.1 17.3 20 17.5 17.7 18.0 18.2 18.4 21 18.6 18.9 19.1 19.3 19.6 22 19.8 20.1 20.3 20.6 20.8 23 21.1 21.3 21.6 21.8 22.1 24 22.4 22.6 22.9 23.2 23.5 25 23.7 24.0 24.3 24.6 24.9 26 25.2 25.5 25.8 26.1 26.4 27 26.7 27.0 27.4 27.7 28.0 28 28.3 28.7 29.0 29.3 29.7 29 30.0 30.4 30.7 31.1 31.5 30 31.8 32.2 32.6 32.9 33.3 References “General chemistry Laboratory Manual for Tennessee State University”, Carlos Lee, Harcourt Brace College Publishers, 1999. “Chemistry”, R. Chang, 7th ed., McGraw-Hill Publishers, 2002. “CRC Handbook of Chemistry and Physics,”R. C. Weast, ed., CRC Press, p. D-193, 1984. Appendix Assembling the Gas Collecting Apparatus Note: An example apparatus will be setup in the room. The following equipment will be needed for the gas-collecting apparatus: a dry 25 x 150mm test tube with a one-hole stopper, a ring stand, a buret and two one-hole rubber stoppers to fit, a leveling bulb, and two pieces of plastic or rubber tubing. The test tube will be the reaction vessel. The reaction vessel will have a one-hole stopper and will be connected via rubber tubing to the upper end of the buret. The buret will be used to measure gas displacement. At the lower end of the buret will be a second piece of tubing connected to a leveling bulb. The leveling bulb is used to keep pressure constant in the buret. Fill the leveling bulb about half full and position it so that the buret graduations are nearly immersed. Check for water leaks, which will lead to errors. Note: In order to obtain useful data from this experiment, the gas-collecting system must be air tight during the reaction and while taking the final buret reading. Any leak in the system will result in a low volume of collected oxygen. 6