TPJ_4123_sm_SupplementalDataLegends
advertisement

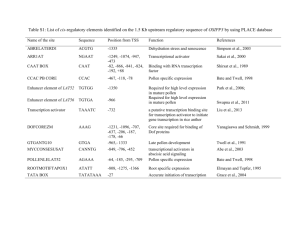
Supplemental Data The SKP1-like Protein SSK1 is Required for Cross-Pollen Compatibility in S-RNase-Based Self-Incompatibility Lan Zhaoa,b,1, Jian Huanga,1, Zhonghua Zhao a,b,1, Qun Lia, Thomas L. Simsc and Yongbiao Xuea,2 a Laboratory of Molecular and Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences and National Center for Plant Gene Research, Beijing 100101, China b c Graduate University of Chinese Academy of Sciences, Beijing 100049, China Department of Biological Sciences and Plant Molecular Biology Center, Northern Illinois University, DeKalb, IL 60115-2861, USA 1 These authors contributed equally to this work 2 Address for correspondence to ybxue@genetics.ac.cn. This file includes: Supplemental Figures 1 to 10 Supplemental Tables 1, 2 and 3 1 Supplemental Figure 1. Molecular identification of four PhSLFs (A) An amino acids sequence alignment of PhSLFs, PiSLFs and their paralogs (PiA134s). PhSLFs were cloned from SI P. hybrida of four S hyplotypes using a homology-based PCR approach. PiSLFs were identified as the pollen S in SI response (Sijacic et al. 2004). Residues of over 80% similarity are shaded in black, those of 60-80% similarity in dark gray and 30-60% similarity in light gray. Numbers show the position of amino acid residues starting from Met. The PhSLFs and PiSLFs share high sequence similarities of over 90%. (B) An unrooted neighbor-joining phylogeny tree of the SLFs and their paralogs was constructed by MEGA 4.1 with default parameter values. Bootstrap values are given at branch nodes. PhSLFs and PiSLFs are also closely related to each other and formed one single clade in the phylogenetic tree, whereas they were diverged from paralogs of PiSLFs, PiA-134-S1, -S2 and -S3. (C) RNA expression analysis of PhSLFs. The RNA levels of PhSLF-S1, S3L and SV in SI P. hybrida of their corresponding S hyplotypes were examined by quantitative RT-PCR using pollen, style and leaf tissues. The results showed that the PhSLFs are pollen-specific genes. Together, these results show that PhSLFs are indeed the orthologs of PiSLFs, which have been demonstrated to be the pollen S gene (Sijacic, et al. 2004), and they could encode the pollen S genes in P. hybrida. Supplemental Figure 2. PhSSK1 accumulates in the mature pollen. Western blot analysis of PhSSK1 during anther development. PS I-VI indicate six developmental stages of P. hybrida flower as represented by the lengths of flower buds of ~2.0 cm, ~3.0 cm, ~4.0 cm, and 5.5 cm, respectively. PS V-VI indicate the flower opening stages, and in PS V the anthers still not open, while in PS VI anthers open and pollen begin to shed. Total proteins of anther or pollen were probed by the anti-PhSSK1 antibody. Tubulin was used as a loading control. 2 Supplemental Figure 3. Amino Acid sequences and the predicted secondary structures of SSK1 proteins. For SSK1 proteins, their secondary structures were predicted through PredictProtein website (http://www.predictprotein.org/). The secondary structure of HsSKP1 has been reported and is shown together with SSK1 proteins (Schulman et al. 2000). Supplemental Figure 4. An unrooted neighbour-joining phylogeny tree of PhSSK1, AhSSK1 and Skp1-like proteins. Fifty-seven deduced Skp1-like proteins are from budding yeast (ScSkp1, AAC49492), human (HsSkp1, AAH20798), Arabidopsis (21 ASKs) (Zhao et al. 2003), rice (26 Skp1-like proteins predicted by TIGR, http://rice.plantbiology.msu.edu), Petunia (3 PiSKs) (Hua and Kao 2006), and other higher plants (FAP1, FAP2, FAP3,(Ingram et al. 1997); NbSkp1, AAO85510; BnSkp1, AAF82795). The neighbour-joining phylogeny tree of these Skp1-like proteins was generated by MEGA 4.1 with default parameter values. PhSSK1 and AhSSK1 form a monophyletic clade as indicated in a dotted line box. Bootstrap values are given at branch nodes. Supplemental Figure 5. Schematic representation of the PhSSK1 RNAi vector (pBI101-LAT52-PhSSK1-RNAi). Lat52 promoter was obtained from S. McCormick (Twell et al. 1991). Supplemental Figure 6. Expression analyses of PhSSK1 in pollen of the T0 transgenic lines. (A). Quantitative RT-PCR analysis of the PhSSK1 RNA levels of the six T0 transgenic lines. PhSKP1-2 was used as a negative control. (B). Western blot analysis of the PhSSK1 expression in the T0 lines. The PhSSK1 protein level of the T0 lines was examined by western blot analysis using total pollen 3 proteins from five T0 lines (B, J2, K4, H6 and M8) (top panel). Tubulin was used as a loading control. The relative PhSSK1 protein amounts in the transgenic lines compared with wild-type pollen were calculated by Quantity One (Bio-Rad) (bottom panel). Supplemental Figure 7. Molecular identification of the style S defective line SOSO. (A) DNA sequence alignment of SO-RNase (M81686) and the S-RNase sequence denoted as SOSO. To identify a stylar S function defective plant, we searched commercially available stocks of self-compatible (SC) P. hybrida and found a self-compatible line with white flowers. To examine whether the SI of its style was normal or not, the S-RNase of the compatible line of SOSO was identified by a homology-based PCR cloning approach. The primers used are listed in Supplemental Table 2. Sequencing alignment showed that this S-RNase was of SO haplotype as described previously (Robbins et al. 2000). Since S-genotyping of progeny derived from the crosses between this SC line and plants with other S-genotypes demonstrated that all progeny carried the S-RNase-SO (see Table 2 and Supplemental Figure 9), this SC line should be homozygous for its SI genes and thus it was referred to as SOSO in this study. It was reported that the S-RNase of SO allele was found to be present in approximately 80% of existing research stocks and commercial cultivars of P. hybrida and is probably a major contributor to self-compatibility observed in many P. hybrida varieties (Robbins, et al. 2000), suggesting that S-RNase-SO is a non-functional allele despite of its RNA expression. (B) Ribonuclease activity and expression of S-RNases in P. hybrida. (a) In-gel assay of ribonuclease activity in Petunia. The arrow indicates active S-RNases present in SI lines. The numbers on the left side indicate the molecular weight (MW) in kD. The result showed that little S-RNase activity could be detected in the total protein of SOSO. (b) Western blot analysis of S-RNase-SO antigen and S-RNases in P. hybrida of SC line (SOSO) and three SI lines (S1S1, S3LS3L and SVSV). Bands marked with asterisk 4 represent a non-specific protein detected by the antibody. The numbers on the left side indicate the molecular weight in kD. Gel stained with coomassieblue was used as a loading control. Western blot was probed by an anti-S-RNase antiserum, which was made using S-RNase-S3L as the antigen and also cross-reacted with the S-RNase-SO antigen expressed in E. coli. The result showed that little S-RNase was detected in the SOSO styles, indicating that the S-RNase-SO protein is unlikely to be expressed. Incidentally, an extra prominent higher molecular weight band also was detected in the stylar proteins of the S3LS3L and SVSV lines but not the S1S1 line. Because S-RNases are known to be differentially glycosylated (Woodward et al. 1989, Woodward et al. 1992), we postulated that S-RNase-S3L and -SV might be more glycosylated than S-RNase-S1. In fact, two potential N-glycosylation sites were found in both S-RNase-S3L and -SV but none in S-RNase-S1 (Supplemental Figure 8). Although further deglycosylation treatment of stylar extracts is required to confirm this, the differential glycosylation of S-RNases could explain our western blot results. Taken together, these results suggested that the SOSO line has a defective stylar S function due to the lack of S-RNase protein expression and could be a suitable line to test whether the reduced fertility of cross compatible pollen observed in the PhSSK1 RNAi transgenic lines was related to pollen tube developmental processes and/or SI responses. Supplemental Figure 8. Predicted glycosylation sites in PhS-RNase-S3L/SV/S1/SO. Potential amino acid sites for glycosylation in PhS-RNase-S3L/SV/S1/SO were predicted by NetNGlyc 1.0 Server and NetOGlyc 3.0 Server and are highlighted in red (Julenius et al. 2005). The amino acids ‘N’ (asparagine) and ‘T’ (threonine) are possible N-glycosylation and O-glycosylation sites, respectively. Supplemental Figure 9. Genotype identification of T2 progeny from the outcrosses of the SOSO wild type compatible line with the T1 transgenic lines as pollen donor. 5 Twenty individuals are shown for each T2 progeny population. T-DNA, S3L-RNase, SV-RNase and SO-RNase were detected by genomic DNA PCR using gene-specific primers. The parents of the each T2 population, the SC wild-type SOSO and the T1 transgenic lines, were used as controls. Supplemental Figure 10. Genotype identification of T2 progeny derived from the outcrosses of the S3LS3L wild type plants with the T1 transgenic lines as pollen donor. Twenty individuals are shown for every T2 progeny population. T-DNA, S3L-RNase and SV-RNase were detected by genomic DNA PCR using gene-specific primers. The parents of the each T2 population, SI wild-type S3LS3L and T1 transgenic lines, were used as controls. Supplemental Table 1. A summary of yeast two-hybrid assays between the Skp1-like proteins and the S-locus F-box proteins of A. hispanicum and P. hybrida. Supplemental Table 2. Pollination analyses of seven independent T0 transgenic lines. Supplemental Table 3. Primers used in this study. 6 Reference Hua, Z. and Kao, T.-H. (2006) Identification and characterization of components of a putative Petunia S-locus F-box-containing E3 ligase complex involved in S-RNase-based self-incompatibility. The Plant cell, 18, 2531-2553. Ingram, G.C., Doyle, S., Carpenter, R., Schultz, E.A., Simon, R. and Coen, E.S. (1997) Dual role for fimbriata in regulating floral homeotic genes and cell division in Antirrhinum. EMBO J, 16, 6521-6534. Julenius, K., Molgaard, A., Gupta, R. and Brunak, S. (2005) Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology, 15, 153-164. Robbins, T.P., Harbord, R.M., Sonneveld, T. and Clarke, K. (2000) The Molecular Genetics of Self-incompatibility in Petunia hybrida. Annals of Botany, 85 (supplement A), 105-112. Schulman, B.A., Carrano, A.C., Jeffrey, P.D., Bowen, Z., Kinnucan, E.R., Finnin, M.S., Elledge, S.J., Harper, J.W., Pagano, M. and Pavletich, N.P. (2000) Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature, 408, 381-386. Sijacic, P., Wang, X., Skirpan, A.L., Wang, Y., Dowd, P.E., McCubbin, A.G., Huang, S. and Kao, T.-H. (2004) Identification of the pollen determinant of S-RNase-mediated self-incompatibility. Nature, 429, 302-305. Twell, D., Yamaguchi, J., Wing, R.A., Ushiba, J. and McCormick, S. (1991) Promoter analysis of genes that are coordinately expressed during pollen development reveals pollen-specific enhancer sequences and shared regulatory elements. Genes Dev, 5, 496-507. Woodward, J.R., Bacic, A., Jahnen, W. and Clarke, A.E. (1989) N-Linked Glycan Chains on S-Allele-Associated Glycoproteins from Nicotiana alata. The Plant cell, 1, 511-514. Woodward, J.R., Craik, D., Dell, A., Khoo, K.H., Munro, S.L., Clarke, A.E. and Bacic, A. (1992) Structural analysis of the N-linked glycan chains from a stylar glycoprotein associated with expression of self-incompatibility in Nicotiana alata. Glycobiology, 2, 241-250. Zhao, D., Ni, W., Feng, B., Han, T., Petrasek, M.G. and Ma, H. (2003) Members of the Arabidopsis-SKP1-like gene family exhibit a variety of expression patterns and may play diverse roles in Arabidopsis. Plant physiology, 133, 203-217. 7