Part 3
advertisement

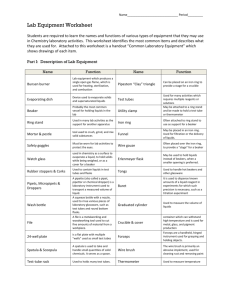
EXPERIMENT 3: TEMPERATURE Introduction: You will learn to work with the Bunsen burner and take temperature measurements with a thermometer. You will apply the graphing principles you have learned to graph a cooling curve as a beaker of hot water loses heat over a period of time. Materials: 250 mL beaker, thermometer, ring stand, iron ring, wire gauze, thermometer holder (obtain from stockroom), Bunsen burner, 2 boiling stones An exercise with the burner and thermometer. Part 1: The "Bunsen" burner is commonly used for laboratory heating operations. There are several types of burners, differing in details of construction, but each has a gas inlet that attaches to a valve on the bench, a gas regulation knob, a tube for mixing air and gas, and an opening near the base of the tube for introduction of air. Find these parts on your burner and make a neat, labeled sketch of the burner on your report sheet. Light the burner by partially closing the air vent and slightly opening the gas regulation knob, turning on the bench gas valve, and bringing a match in toward the top of the burner from the side, touching the top of the tube. Observe the flickering orange flame. This kind of flame is called a “luminous flame”. Open the air hole about half way and open the gas regulation knob until the flame is about 8 to 10 cm high and the flame is blue. A blue flame is hotter than an orange flame. Once again close the air hole at the base of the tube. Using crucible tongs, hold an evaporating dish about 5 cm above the top of the tube. Observe what happens on the dish. Part 2: In this part of the experiment you will work alone. Each student must check out a thermometer, a thermometer holder, and a stopwatch from the stockroom or from your instructor. Set up a ring stand with an iron ring and wire gauze. Weigh a 250 mL beaker with two boiling stones. Measure out approximately 150 to 175 mL of deionized water in a graduated cylinder then add it to the beaker. Re-weigh the beaker with water. Record all of your data on the report sheet. Calculate the weight of water. Place the beaker of water on the wire gauze and set up the Bunsen burner next to the ring stand. Measure the temperature of the water to the nearest 0.1oC (this is room temperature). note – you may check out a thermometer holder to use instead of the utility clamp shown Position the thermometer in the water and heat the water boiling. Do not allow the thermometer to touch the sides bottom of the beaker. Keep the thermometer suspended. Practice adjusting the flame, by making it yellow, then blue, then blue with a sharp blue cone inside the lighter flame. to or faintly blue Theory states that water boils at a constant temperature until all of the liquid is converted into its gaseous state. You will test this statement by measuring the temperature of boiling water for 15 minutes in 5 minute intervals. Allow the water to boil for 5 minutes and while the flame is still active, measure the temperature of the water then repeat this measurement after another 5 minutes. Look up the boiling point of water and compare that value to your experimentally determined value. This technique is referred to as “calibration of the thermometer”. Is your thermometer accurate? Record the temperature of boiling water again 5 minutes later. Has it changed? The boiling point of any pure substance is characteristic of that material and can be used to identify that substance. Record your observations Part 3: Turn off the burner. Clamp the thermometer to the ring stand and submerge the tip of the thermometer in the cooling water. When the water has reached about 85oC, start recording the temperature at 1minute intervals. Use a glass rod to gently stir the water. Use the stopwatch to keep track of time. Record the temperatures to the nearest 0.1oC. Continue making measurements for 30 minutes or until the temperature reaches 35oC. Graph this data, either by hand or by using Excel, as temperature vs. elapsed time to create a cooling curve. Record time in minutes on the x-axis and temperature in oC on the y-axis. Draw a smooth curve passing through most of the points. Attach the graph to the report sheet. Do not forget to title the graph. Part 4: Once the water has returned to room temperature, record the temperature again. It must match the first temperature reading before you heated the beaker of water. Weigh the beaker of water. Calculate the amount of water missing. Part 5: Use the Fahrenheit – Celsius conversion formulas to set up a table of several sets of temperatures in oF and oC ranging from –50oC up to 100oC. o F = 1.8 oC + 32 C = oF – 32 1.8 o Your instructor will require you to graph the values in the table provided using either Excel or by hand. Use the x-axis for the Celsius degrees and the y-axis for the Fahrenheit degrees. Find the slope of the graph. Attach both graphs to the report sheets along with any notebook copy pages EXPERIMENT 3: Report Sheet A STUDY OF TEMPERATURE: Name: ______________________ section ________________ Part 1: Observations Sketch of burner (Label parts): 1. What color is the flame when the oxygen intake has been minimized? ____________ 2. What color is the hottest flame? ________________________ 3. What deposits on the bottom of the evaporating dish when a luminous yellow flame applied to the dish? _________________ 4. What do you think is the cause of the deposit on the evaporating dish? 5. (Optional question): Write a chemical equation describing this event (combustion). Part 2: Data Weight of beaker and water __________________ Weight of beaker __________________ Weight of water __________________ Room Temperature of water __________________ Calibration of the thermometer: Temperature of boiling water (1st) __________________ Temperature of boiling water (2nd) __________________ Temperature of boiling water (3rd) __________________ 1. What is the theoretical temperature of boiling water from handbook? _________ 2. Is your thermometer accurate? ___________ is Part 3: Data for cooling water Elapsed Time in minutes Temperature (oC) Part 4: Data and observations Temperature of water before heating __________________ Weight of beaker and water once cooled __________________ How much water was lost during the boiling process? __________________ Explain what happened to the water that was lost. Part 5: Temperature conversions Fill in the table below with some temperatures in Celsius and their equivalent values in Fahrenheit degrees. Celsius Fahrenheit – 50. 0. 75 100. Plot the Celsius degrees on the x-axis and the Fahrenheit degrees on the y-axis. Pick two points on the graph that are fairly far apart to calculate the slope. Do not use the points which you have already plotted. Find 2 new points arbitrarily, on the line. Show set-up of slope calculation or how it was obtained from the computer-generated equation of the line. Comment on the value you obtained for the slope of the graph. At what temperature is the Celsius reading the same as the Fahrenheit? ________ (Show Calculations)