Determination of the Molar Mass of an Unknown Gas from Density
advertisement

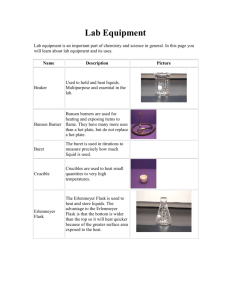
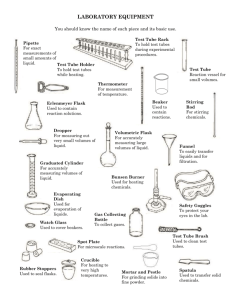
Title: Determination of the Molar Mass of an Unknown Gas from Density Materials: One large test tube 5 ml of ice cold water Generating tube ( rubber stopper, glass bend, and rubber tubing) 250ml beaker Alka-Seltzer ( 1 tablet) Balance 250ml Erlenmeyer flask 100ml graduated cylinder Large container half filled with water Procedure: Gather all materials and return to lab station. Add 5ml of ice cold water to the large test tube. Place the test tube with water into the beaker. Place the Alka-Seltzer and beaker containing test tube and water onto the pan of the balance and record the mass to 0.01g in the data section. Completely fill an Erlenmeyer flask with water Measure the exact volume of the Erlenmeyer flask by pouring the water into the graduated cylinder and record the volume to 0.1ml in the data section. Refill the Erlenmeyer flask completely. Place your hand over the mouth of the flask and carefully invert it into the container with water making sure there are no air bubbles in the flask. Insert the rubber tubing into the flask. Place the Alka-Seltzer into the test tube and cap the stopper immediately with the stopper. Let the reaction run to completion (approximately 7-10 minutes). Remove the tubing from the flask and unstop the test tube. Using the balance, record the mass of the test tube and remaining solution to 0.01g in the data section. Place your hand over the mouth of the flask and remove the flask from the large container. Measure the volume of remaining water in the flask using the graduated cylinder and record the volume to 0.1L in the data section. Data: 1. 2. 3. 4. Mass of beaker, test tube, water, and Alka-Seltzer before mixing _____g Initial volume of water in Erlenmeyer flask ___________mL Mass of beaker, test tube, and solution after mixing ___________g Final volume of remaining water in Erlenmeyer flask ____________mL Results: 1. Calculate the difference in mass. 2. Calculate the difference in mL and convert to Liters. 3. Calculate the density of the unknown gas. 4. Calculate the molar mass of the unknown gas from density. 5. Calculate the percent error of molar mass. 6. Knowing the molar mass of the gas, how could you determine the mass lost in the reaction from volume only? 7. Calculate the percent error of mass.