Methylphenidate in Children SCP
advertisement

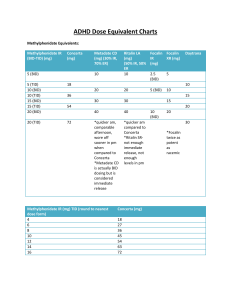
SCP 4 SHARED CARE PROTOCOL Methylphenidate Clinical Indication: Attention Deficit Hyperactivity Disorder (ADHD) in children (aged 6 to 18 ) Version 1: Oct 2014 due for review: Nov 2016 Introduction Attention-Deficit Hyperactivity Disorder (ADHD) is diagnosed if the three clinical features -inattention, over-activity and impulsiveness - have been present from an early age, persist in more than one situation (e.g. at home and in school) and impair function. The diagnosis must be made following a comprehensive assessment by an appropriate child psychiatrist and/or a paediatrician with special interest and training in this field. The assessment and management of this condition has been reviewed by SIGN Guideline No. 112, October 2009 and NICE Quality Standard 39, July 2013, Drug therapy with Methylphenidate is only one part of the package of care for children with ADHD, which includes behavioural and educational interventions. Shared Care A shared care protocol is used to facilitate the sharing of care and transfer of prescribing. This would usually take place once the patient’s condition is stable; the patient is demonstrably benefiting from the treatment and is free from any significant side effects. GPs should only take on the prescribing when they are confident in the use of the drug, in the context of the protocol. Contingency plans must be in place to enable the patient to receive the recommended treatment, should the GP decline to prescribe. Indication for Therapy Methylphenidate (Ritalin®/ Medikinet® /Equasym XL®/Concerta XL®/ Medikinet XL®) is licensed as part of a comprehensive treatment programme for ADHD when remedial measures alone prove insufficient. Treatment must be initiated under the supervision of a child psychiatrist and/or a paediatrician with special interest and training in this field. The drug is not licensed for children less than six years of age, as a result, this shared care protocol applies to children 6 years old and above, until care is transferred from Paediatric and Young Persons Services to Adult Psychiatric Services. Preparations Available Methylphenidate is available in the following formulations: Ritalin® - 10mg scored tablet (30 tablet pack) Medikinet® - 5mg, 10mg and 20mg scored tablet (30 tablet pack) Concerta XL® - 18mg, 27mg and 36mg modified release capsule (30 capsule pack) Equasym XL® - 10mg, 20mg and 30mg modified release capsule (30 capsule pack) Medikinet XL® - 5mg, 10mg, 20mg, 30mg and 40mg modified release capsule (28 capsule pack) Methylphenidate preparations can be obtained from the wholesaler by a community pharmacist. It is controlled by the Misuse of Drugs Act 1971 and is therefore subject to the regulations for controlled drugs in relation to the requirements for safe custody and stating the quantity to be dispensed in both words and figures. From June 2006 prescriptions for Controlled Drugs must be dispensed within 28 days of the date on the prescription. Recommended Dosage and Administration Doses are started at 5mg once or twice daily or for children over 6 years can be initiated on equivalent dose of longer acting preparation and increased by the psychiatrist/paediatrician. If improvement is not observed after appropriate dosage adjustment the drug will be discontinued. The maximum recommended dose for methylphenidate is 60mg daily (in 2 or 3 divided doses). In some children a rebound hyperactivity may occur as the effect of the drug wears off in the evening. Dividing the doses to include a small evening dose of immediate release preparation may eliminate this difficulty. Modified release preparations -1Created by amcvean 17/02/2016 Concerta XL® may be used once daily in the morning, as indicated above, swallowed whole, CANNOT be chewed, opened, divided or crushed. Patients can be converted from standard methylphenidate preparations as described in the Summary of Product Characteristics. The dosage can be adjusted in 9mg increments from an initial 18mg to a maximum of 54mg once daily in the morning. Equasym XL® should be given once daily in the morning before breakfast. Patients can be converted from standard methylphenidate preparations as described in the Summary of Product Characteristics. The dosage can be adjusted in 10mg increments to a maximum of 60mg daily. Medikinet XL® should be given once daily in the morning with or after breakfast. Patients can be converted from standard methylphenidate preparations as described in the Summary of Product Characteristics. The dosage can be adjusted according to response to a maximum of 60mg daily. Both Equasym XL® and Medikinet XL® capsules may be swallowed whole with the aid of liquids and must NOT be chewed or crushed. Alternatively, the capsule may be opened and the capsule contents sprinkled onto a small amount (tablespoon) of apple sauce and given immediately. Shared Care Responsibilities Aspects of Care for which the Specialist is responsible Assessment and diagnosis of children with ADHD Before treatment, screen all for problems with blood pressure or heart rate and take a family history of cardiac and cardiovascular disease. An ECG may be required. To prescribe the first dose during the initiation of trial Liaison with GP to agree to share the patient’s care Making suitable arrangements with the patient in the event the practice are not willing to prescribe. Patient monitoring - initially 3 monthly, then 6 monthly in the longer term. This includes height, weight and blood pressure and pulse. Discontinuation - advising GP when Methylphenidate should be discontinued for patients receiving the drug long term. The specialist will provide necessary supervision and support during the drug discontinuation phase Continuing supply of Methylphenidate for children under 6 years old. If Methylphenidate is continued beyond 17 years then care should be transferred from CAMHS to Adult Psychiatric Services as appropriate Communication to ensure GP is informed of any changes in treatment e.g. patient monitoring, dose alteration etc Aspects of Care for which the General Practitioner is responsible To prescribe the drug if willing to do so under the instruction from the Specialist If unwilling to prescribe the drug, to ask if any colleagues are willing to prescribe. If the practice is unwilling to prescribe to relay this decision back to the Specialist in a timely manner for alternative arrangements to be established. Liaison with the Paediatrician/Psychiatrists regarding any complications of treatment Reporting of adverse reactions to Yellow Card Scotland is the responsibility of both the Consultant and GP https://yellowcard.mhra.gov.uk/ Adverse Effects The following have been reported: Insomnia, nervousness (>10%) Decreased appetite. Occasional abdominal pain, nausea and vomiting (alleviated with concomitant food intake). Headaches, arthralgia. Emotional lability. Temporary growth retardation may occur during prolonged therapy - monitor height and weight. Changes in blood pressure and heart rate (usually increased). Drug Interactions Warfarin- may increase the anticoagulant effect Anticonvulsants- phenytoin and phenobarbitone levels may be increased -2Created by amcvean 17/02/2016 Tricyclic antidepressants- plasma levels may be Increased Alcohol- can increase CNS effects of methylphenidate No known interactions with antibiotics, simple analgesics and antihistamines commonly prescribed for children Contra-Indications Contra-indicated in patients with marked anxiety disorders, psychosis, cardiovascular disease (including hypertension), hyperthyroidism, glaucoma, and in pregnancy (especially during first trimester) Contra-indicated in Tourette’s syndrome but is used with caution by specialists. Any prescribing in this situation would be unlicensed. Hypersensitivity or intolerance to methylphenidate or excipients. Concerta XL® should not be used in patients with severe gastrointestinal tract narrowing or dysphagia or significant difficulty with swallowing tablets Precautions Patients with a history of epilepsy and in patients with a tic disorder. Medication in methylphenidate m/r (Concerta XL®) is contained within a non-deformable, nonabsorbable shell which is eliminated unchanged from the body in the patient’s stool. Contact Points Borders CAMHS Primary Care Prescribing Team BGH Pharmacy Dr Diana Leaver, Community Paediatrician West Lothian ADHD Service Tel: 01750 23715 Tel: 01896 827708 Tel: 01896 826783 Tel: 01896 826686 Tel: 01506 419666 ext 2734 This information was prepared by the Consultants in the Department of Child and Adolescent Mental Health Service and the Pharmacists at the Royal Edinburgh Hospital through liaison with the General Practice Prescribing Committee, the Paediatric & Neonatal Drug and Therapeutics Committee, Lothian University Hospitals Division, and the Hospital and Specialist Services Medicines Committee, NHS Lothian. Used and adapted with permission. -3Created by amcvean 17/02/2016