CHAPTER 1 - Figshare
advertisement

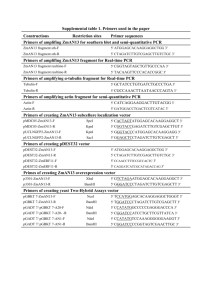
Text S1 Friedreich's Ataxia (GAA)n•(TTC)n Repeats Strongly Stimulate Mitotic Crossovers in Saccharomyces cerevisae. Wei Tang1, Margaret Dominska1, Patricia W. Greenwell1, Zachary Harvanek1, Kirill S. Lobachev2, Hyun-Min Kim2,3, Vidhya Narayanan2, Sergei M. Mirkin4, and Thomas D. Petes1* 1Department of Molecular Genetics and Microbiology, Duke University, Durham, NC 27710, USA. 2School of Biology and Institute for Bioengineering and Bioscience, Georgia Institute of Technology, Atlanta, GA 30332, USA. 3Present address: Department of Genetics, Harvard Medical School, Boston, MA 02115. 4Department of Biology, Tufts University, Medford, MA 02155, USA. *Corresponding author Department of Molecular Genetics and Microbiology Duke University Medical Center Durham, NC 27710, USA Strain constructions Lee et al. [1] previously described the construction of two haploid strains derived from different genetic backgrounds: PSL2 derived from W303a [2] and PSL5 derived from YJM789 [3]. Both PSL2 and PSL5 have the wild-type RAD5 gene. These strains have diverged to the extent of about six single-nucleotide polymorphisms (SNPs) per kb [3]. Diploids formed by crossing these strains, therefore, are heterozygous for many markers that can be used to map mitotic recombination events. Genotypes of haploids used in strain constructions are in Table S1, oligonucleotides used in constructions are in Table S2, and diploid genotypes are in Table S3. A precursor strain to PSL2 was the haploid MAB1 ([4]; genotype: MATcan1-100 leu2-3,112 his3-11,15 ura3-1 ade2-1 trp1-1 V9229::HYG V261553::HIS3 RAD5). The LYS2 gene on chromosome II was removed from MAB1 by the delitto perfetto procedure [5]. First, the primers LYS2-DEL-F and LYS2-DEL-R were used to amplify a KANMX-URA3-cassette contained in plasmid pCORE [5]. The resulting fragment was transformed into MAB1, generating strain HMK151. We subsequently transformed HMK151 with the partially-complementary oligonucleotides DEL-CORE-F and DELCORE-R, selecting for a 5-FOAR derivative that was also geneticin-sensitive, resulting in the strain HMK160. HMK160 was transformed with a PCR fragment generated by amplifying DNA from the plasmid pFL39LYS2 with oligonucleotides LYS2-INS-F and LYS2-INS-R; the plasmid pFL39LYS2 contains a wild-type LYS2 gene inserted as an EcoRI-HindIII fragment in the vector pFL39 [6]. The resulting Lys+ strain, HK9, had a 4.5 kb LYS2 insertion between bases 115902 and 115903 of chromosome V (the GEA2-URA3 intergenic region). HK9 was then transformed with a DNA fragment generated by BsrGI digestion of pFL39LYS2::CORE; this plasmid is a derivative of 2 pFL39LYS2 in which the 3.2 kb KANMX-URA3 cassette is inserted in the BamHI site within the LYS2 gene. In the resulting transformant (HK10), the 3.2 kb KANMX-URA3 cassette is integrated into the LYS2 gene on chromosome V approximately 3.2 kb from the beginning of the LYS2 coding sequence. The next step in the construction was to replace the KANMX-URA3 cassette with (GAA)n(TTC)n tracts of different sizes and orientations. HK10 was transformed with PCR fragments containing the tracts, selecting 5-FOAR derivatives and screening for those that were also geneticin-sensitive. These fragments were generated by amplification of genomic DNA from previously constructed yeast strains containing (GAA)n(TTC)n tracts of various sizes [7] using the primers LYS2-TRACT-F and LYS2TRACT-R. The resulting transformed strains were HK11-(GAA)20, HK17-(GAA)230, HK20-(TTC)20, and HK26-(TTC)230. The tracts in these strains are located about 1.2 kb centromere-distal to URA3 on chromosome V. All transformants were checked to be sure that the tracts were of the correct size by PCR using the primers AAG-F and AAGR. We constructed URA3 derivatives of HK11-(GAA)20, HK17-(GAA)230, HK20-(TTC)20, and HK26-(TTC)230 (MD500, MD501, MD502, and MD503, respectively) by transformation of the haploids with a PCR fragment resulting from amplification of genomic MAB10 DNA [4] with the primers wtURA3F and wtURA3R. As described below, genomic DNA from strains MD500-MD503 was used to construct the strains WXT10-WXT13, and WXT30-WXT33. For strains MD500-503, the (GAA)n(TTC)n tracts are embedded within the 4.2 kb LYS2 gene. We constructed derivatives of these strains in which the tracts were 3 inserted into a truncated lys2 gene about 1.1 kb in size located between GEA2 and URA3 at the same position as the longer insertion. To construct WXT10, we amplified genomic DNA from MD502 with the primers AAG-BIG1-F and AAG-BIG1-R. The resulting fragment, which contains the (GAA)n(TTC)n tract and the closely-linked URA3 gene, was transformed into PSL5, selecting Ura+ transformants. A similar procedure with the same primers was used to construct WX11-WXT13. For these strains, PSL5 was transformed with a PCR fragment in which the sources of genomic DNA used for the amplifications were MD503 for WXT11, MD500 for WXT12, and MD501 for WXT13. The WXT30-WXT33 strains were derived from PSL2 in two steps. First, using the same primers that were employed in constructing WXT10-13, we generated PCR fragments that contained the (GAA)n(TTC)n tracts and URA3; these fragments were used to transform PSL2 to Ura+. The sources of genomic DNA used in these constructions were: MD502 for WXT20, MD503 for WXT21, MD500 for WXT22, and MD501 for WXT23. We then constructed ura3 derivatives of WXT20, 21, 22, and 23 (WXT30, 31, 32, and 33, respectively) by transformation of WXT20-23 with a PCRgenerated fragment with a mutant ura3 gene; this fragment was the result of amplification of PSL2 genomic DNA with the primers URA3-F and URA3-R. Uratransformants were selected with medium that contained 5-fluoro-orotate. The haploids WXT30-33 were crossed to the haploids WXT10-13 in various combinations to generate the diploids WXTMD40-46 (Table S3). The haploid strains MD510 and MD512 are isogenic derivatives of PSL2 with insertions of (GAA)230 and (TTC)230 near URA3. The context of the tract insertion is identical to that in strains MD500-MD503. MD510 was a spore of the diploid MD506 4 (MAB4 x MD501); MAB4 has been previously described [4]. MD512 was a spore of the diploid MD508 (MAB4 x MD503). MD510 and MD512 are isogenic except for the orientation of the trinucleotide tract (Table S1). 5 References 1. Lee PS, Greenwell PW, Dominska M, Gawel M, Hamilton M, et al. (2009) A finestructure map of spontaneous mitotic crossovers in the yeast Saccharomyces cerevisiae. PLoS Genet 5: e1000410. 2. Thomas BJ, Rothstein R (1989) Elevated recombination rates in transcriptionally active DNA. Cell 56: 619-630. 3. Wei W, McCusker JH, Hyman RW, Jones T, Ning Y, et al. (2007) Genome sequencing and comparative analysis of Saccharomyces cerevisiae strain YJM789. Proc Natl Acad Sci U S A 104: 12825-12830. 4. Barbera MA, Petes TD (2006) Selection and analysis of spontaneous reciprocal mitotic cross-overs in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 103: 12819-12824. 5. Storici F, Resnick MA (2006) The delitto perfetto approach to in vivo site-directed mutagenesis and chromosome rearrangements with synthetic oligonucleotides in yeast. Methods Enzymol 409: 329-345. 6. Bonneaud N, Ozier-Kalogeropoulos O, Li GY, Labouesse M, Minvielle-Sebastia L, et al. (1991) A family of low and high copy replicative, integrative and singlestranded S. cerevisiae/E. coli shuttle vectors. Yeast 7: 609-615. 7. Kim HM, Narayanan V, Mieczkowski PA, Petes TD, Krasilnikova MM, et al. (2008) Chromosome fragility at GAA tracts in yeast depends on repeat orientation and requires mismatch repair. Embo J 27: 2896-2906. 8. Mortimer RK, Schild D (1981) Genetic mapping in Saccharomyces cerevisiae. In: Strathern JN, Jones EW, Broach JR, editor. The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. Cold Spring Harbor, NY: Cold Spring Harbor Press. pp. 11-26. 6