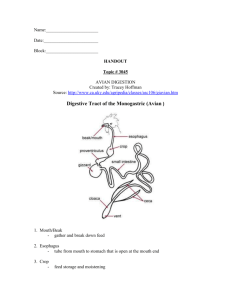
Digestion and the Digestive System
advertisement

Biochemistry of Digestion, Absorption and Detoxification 27/9-9/10/2011 1. 2. 3. 4. 5. 6. Lecture 1: Introduction to the Biochemistry of Digestion, absorption and detoxification. Lecture 2: Digestion and Digestive secretion from mouth and stomach Lecture 3: Digestive secretion from pancreas and liver Lecture 4: Detoxification in the liver Lecture 5: Secretion and absorption in the small intestine Lecture 6: Secretion and absorption in the large intestine Aim and objective of the above six lectures is to understand: The biochemistry and mechanism of digestion of food 1. The absorption of basic nutrients 2. The detoxification mechanism References: 1. "Biochemistry" by Lubert Stryer (textbook) 2. "Textbook of Biochemistry with Clinical Correlations" by T.M.Devlin (additional reading) 3. "Lippincott's Illustrated Reviews in Biochemistry" by P.C.Champe, R.A.Harvey and D.R.Ferrier (additional reading) 4. "Harper's Biochemistry" by R.K.Murray, D.K.Granner, P.A. Mayes and V.W.Rodwell. (additional reading) Prof. Dr.H.D.El-Yassin1 2011 LECTURE 1 Tuesday 27/9/2011 Introduction to the Biochemistry of Digestion, Absorption and Detoxification Introduction Digestion is the chemical breakdown of large food molecules into smaller molecules that can be used by cells. The breakdown occurs when certain specific enzymes are mixed with the food. Enzymes involved in Digestion polysaccharides maltose proteins peptides fats fatty acids and glycerol glucose amino acids Prof. Dr.H.D.El-Yassin2 2011 LECTURE 1 Tuesday 27/9/2011 Review of Food Chemistry The diet of any animal contains hundreds if not thousands of different molecules, but the bulk of the ingested nutrients are in the form of huge macromolecules that cannot be absorbed into blood without first being reduced to much simpler and smaller forms. The most important enzymatic reaction in digestion of foodstuffs is hydrolysis - the breaking of a chemical bond by the addition of a water molecule. Proteins Proteins are polymers of amino acids linked together by peptide bonds. Chain length varies tremendously and many dietary proteins have been modified after translation by addition of carbohydrate (glycoproteins) or lipid (lipoprotein) moieties. Very short proteins, typically 3 to 10 amino acids in length, are called peptides. Lipids Fatty acids are present in only small amounts in animal and plant tissues, but are the building blocks of many important complex lipids. True fatty acids possess a long hydrocarbon chain terminating in a carboxyl group. Nearly all fatty acids have an even number of carbons and have chains between 14 and 22 carbons in length. The principle differences among the many fatty acids are the length of the chain (usually 16 or 18 carbons) and the positions of unsaturated or double bonds. The most abundant storage form of fat in animals and plants, and hence the most important dietary lipid, is triglyceride. A molecule of triglyceride is composed of a molecule of glycerol in which each of the three carbons is linked through an ester bond to a fatty acid. Triglycerides cannot be efficiently absorbed, and are enzymatically digested by pancreatic lipase into a 2monoglyceride and two free fatty acids, all of which can be absorbed. Other lipases hydrolyse a triglyceride into glycerol and three fatty acids. Prof. Dr.H.D.El-Yassin3 2011 LECTURE 1 Tuesday 27/9/2011 Carbohydrates 1. Monosaccharides or simple sugars are either hexoses (6-carbon) like glucose, galactose and fructose, or pentoses (5-carbon) like ribose. These are the breakdown products of more complex carbohydrates and can be efficiently absorbed across the wall of the digestive tube and transported into blood. 2. Disaccharides are simply two monosaccharides linked together by a glycosidic bond. The disaccharides most important in nutrition and digestion are: lactose or "milk sugar": glucose + galactose sucrose or "table sugar": glucose + fructose maltose: glucose + glucose Oligosaccharides are relatively short chains of monosaccharides which typically are intermediates in the breakdown of polysaccharides to monosaccharides. 3. Polysaccharides : Starch is a major plant storage form of glucose. It occurs in two forms: alpha-amylose, in which the glucoses are linked together in straight chains, and amylopectin, in which the glucose chains are highly branched. Except for the branch points of amylopectin, the glucose monomers in starch are linked via alpha(1->4) glycosidic bonds, which, in the digestive tract of mammals, are hydrolyzed by amylases. Cellulose is the other major plant carbohydrate. It is the major constituent of plant cell walls, and more than half of the organic carbon on earth is found in cellulose. Cellulose is composed on unbranched, linear chains of D-glucose molecules, linked to one another by beta(1>4) glycosidic bonds, which no vertebrate has the capacity to enzymatically digest. Glycogen is the third large polymer of glucose and is the major animal storage carbohydrate. Like starch, the glucose molecules in glycogen are linked together by alpha(1->4) glycosidic bonds. Prof. Dr.H.D.El-Yassin4 2011 LECTURE 1 Tuesday 27/9/2011 The process of digestion produces glucose, amino acids, glycerol, and fatty acids (see above). The energy in glucose is used to produce ATP via the reactions of glycolysis, cellular respiration, and the electron transport system (see diagram below). The body uses amino acids to construct proteins. Excess amino acids can be used to synthesize pyruvate, acetyl CoA, and alpha ketogluterate, which enters the Krebs cycle. Glycerol and fatty acids can be converted to pyruvate and Acetyl CoA and then enter cellular respiration. Mouth Chewing breaks food into smaller particles so that chemical digestion can occur faster. Enzymes: Salivary amylase breaks starch (a polysaccharide) down to maltose (a disaccharide). Bicarbonate ions in saliva act as buffers, maintaining a pH between 6.5 and 7.5. Mucins (mucous) lubricate and help hold chewed food together in a clump called a bolus. Prof. Dr.H.D.El-Yassin5 2011 LECTURE 1 Tuesday 27/9/2011 Stomach The stomach stores up to 2 liters of food. Gastric glands within the stomach produce secretions called gastric juice. The muscular walls of the stomach contract vigorously to mix food with gastric juice, producing a mixture called chyme. Gastric juice Pepsinogen is converted to pepsin, which digests proteins. Pepsinogen production is stimulated by the presence of gastrin in the blood. HCl Hydrochloric acid (HCl) converts pepsinogen to pepsin which breaks down proteins to peptides. HCl maintains a pH in the stomach of approximately 2.0. It also dissolves food and kills microorganisms. Mucous protects the stomach from HCl and pepsin. Secretion of Gastric Juice: Gastrin is a hormone that stimulates the stomach to secrete gastric juice. Duodenum The duodenum is the first part of the small intestine. Chyme enters in tiny spurts. At this point, proteins and carbohydrates are only partially digested and lipid digestion has not begun. Pancreas The pancreas acts as an exocrine gland by producing pancreatic juice which empties into the small intestine via a duct. The pancreas also acts as an endocrine gland to produce insulin. Pancreatic Juice Pancreatic juice contains sodium bicarbonate which neutralizes the acidic material from the stomach. Pancreatic amylase digests starch to maltose. Trypsin and Chymotrypsin digest proteins to peptides. Like pepsin (produced in the stomach), they are specific for certain amino acids, not all of them. They therefore produce peptides. Lipase digests fats to glycerol and fatty acids. Prof. Dr.H.D.El-Yassin6 2011 LECTURE 1 Tuesday 27/9/2011 Liver The liver produces bile which is stored in gallbladder and sent to the duodenum through a duct. Bile emulsifies fats (separates it into small droplets) so they can mix with water and be acted upon by enzymes. Other Functions of the Liver The liver detoxifies blood from intestines that it receives via the hepatic portal vein. The liver stores glucose as glycogen (animal starch) and breaks down glycogen to release glucose as needed. This storage-release process maintains a constant glucose concentration in the blood (0.1%). If glycogen and glucose run short, proteins can be converted to glucose. It produces blood proteins. It destroys old red blood cells and converts hemoglobin from these cells to bilirubin and biliverdin which are components of bile. Ammonia produced by the digestion of proteins is converted to a less toxic compound (urea) by the liver. Hormones Involved in Digestion 1. Gastrin: The presence of food in the stomach stimulates specific receptors which in turn stimulates endocrine cells in the stomach to secrete the hormone gastrin into the circulatory system. Gastrin stimulates the stomach to secrete gastric juice. 2. Secretin: Secretin is produced by cells of the duodenum. It’s production is stimulated by acid chyme from stomach. It stimulates the pancreas to produce sodium bicarbonate, which neutralizes the acidic chyme. It also stimulates the liver to secrete bile. 3. CCK (cholecystokinin): CCK production is stimulated by the presence of food in the duodenum. It stimulates the gallbladder to release bile and the pancreas to produce pancreatic enzymes. 4. GIP (Gastric Inhibitory Peptide):Food in the duodenum stimulates certain endocrine cells to produce GIP. It has the opposite effects of gastrin; it inhibits gastric glands in the stomach and it inhibits the mixing and churning movement of stomach muscles. This slows the rate of stomach emptying when the duodenum contains food. Prof. Dr.H.D.El-Yassin7 2011 LECTURE 1 Tuesday 27/9/2011 Small Intestine The small intestine is approximately 3 m long. Like the stomach, it contains numerous ridges and furrows. In addition, there are numerous projections called villi that function to increase the surface area of the intestine. Individual villus cells have microvilli which greatly increase absorptive surface area. The total absorptive surface area is equivalent to 500 or 600 square meters. Each villus contains blood vessels and a lacteal (lymph vessel). Peptidases and maltase are embedded within the plasma membrane of the microvilli. Peptidases complete the digestion of peptides to amino acids. Maltase completes the digestion of disaccharides. Absorption: The Large Intestine: It functions in three processes: Recovery of water and electrolytes from ingesta: By the time ingesta reaches the terminal ileum, roughly 90% of its water has been absorbed, but considerable water and electrolytes like sodium and chloride remain and must be recovered by absorption in the large gut. Formation and storage of feces: As ingesta is moved through the large intestine, it is dehydrated, mixed with bacteria and mucus, and formed into feces. Microbial fermentation: The large intestine of all species teems with microbial life. Those microbes produce enzymes capable of digesting many of molecules that to vertebrates are indigestible, cellulose being a premier example. Absorption: water, sodium ions and chloride ions Secretion: bicarbonate ions and mucus Prof. Dr.H.D.El-Yassin8 2011 LECTURE 1 Tuesday 27/9/2011 Summary of Digestive Enzymes The digestive enzymes in the table below are summarized according to type of food that they digest. FOOD TYPE ENZYME SOURCE PRODUCTS CARBOHYDRATES Salivary amylase Pancreatic amylase Maltase Salivary glands Pancreas Small intestine Maltose Maltose Glucose PROTEINS Pepsin Trypsin Peptidases Stomach mucosa Pancreas Intestinal mucosa Peptides Peptides Amino acids FATS Lipase Pancreas Fatty acids and glycerol The table below shows digestive enzymes grouped by source of the enzyme. SOURCE ENZYME FOOD PRODUCT MOUTH (salivary glands) Salivary amylase Polysaccharides Maltose STOMACH Pepsin Proteins Peptides PANCREAS Pancreatic amylase Trypsin Lipase Polysaccharides Proteins Fats Maltose Peptides Fatty acids and glycerol SMALL INTESTINE Maltase Peptidases Maltose Peptides Glucose Amino acids Prof. Dr.H.D.El-Yassin9 2011 LECTURE 2 Thursday 29/9/2011 Digestion and Digestive Secretion from Mouth and Stomach The Mouth Complex food substances taken by animals must be broken down into simple, soluble and diffusible substances before they can be absorbed into the body. In the mouth, salivary glands secrete αamylase, which digests starch into small segments of multiple sugars and into the individual soluble sugars. Salivary glands also secrete lysozyme, which kills bacteria but is not classified as a digestive enzyme. The Stomach Foodstuffs entering the stomach have been, crushed and reduced in size by mastication, with saliva. The stomach provides four basic functions that assist in the early stages of digestion and prepare the ingesta for further processing in the small intestine: 1. It serves as a short-term storage reservoir, allowing a rather large meal to be consumed quickly and dealt with over an extended period. 2. It is in the stomach that substantial chemical and enzymatic digestion is initiated, particularly of proteins. 3. Vigorous contractions of gastric smooth muscle mix and grind foodstuffs with gastric secretions, resulting in liquefaction of food, a prerequisite for delivery of the ingesta to the small intestine. 4. As food is liquefied in the stomach, it is slowly released into the small intestine for further processing. If the lining of the stomach is examined with a hand lens, one can see that it is covered with numerous small holes. These are the openings of gastric pits which extend into the mucosa as straight and branched tubules, forming gastric glands. Prof. Dr.H.D.El-Yassin 10 2011 LECTURE 2 Thursday 29/9/2011 Four major types of secretory epithelial cells cover the surface of the stomach and extend down into gastric pits and glands: 1. Mucous cells: secrete an alkaline mucus that protects the 2. epithelium against shear stress and acid 3. Parietal cells: secrete hydrochloric acid. 4. Chief cells: secrete pepsin, a proteolytic enzyme 5. G cells: secrete the hormone gastrin Prof. Dr.H.D.El-Yassin 11 2011 LECTURE 2 Thursday 29/9/2011 Gastric secretions 1. Mucosal Protection Mucus layer on gastric surface forms a mucosal barrier to damage against several forms of potential injury to the gastric mucosa. 1. A gel 0.2mm thick; 80% CHO; 20% protein 2. Secreted by neck cells, surface epithelium 3. Can be cleaved by pepsin, so continual production is required 4. Release is stimulated by acetylcholine from nerve endings 5. Also rich in bicarbonate a. HCO3- content creates a "micro-environment" around surface cells to prevent acid damage b. HCO3- secretion is inhibited by adrenergic input (prominent in stress) 2. Acid Secretion Hydrochloric acid is secreted from parietal cells into the lumen where it establishes an extremely acidic environment. This acid is important for activation of pepsinogen and inactivation of ingested microorganisms such as bacteria. 2.1. Function of Gastric acid 1. To kill micro-organisms: (but H. pylori survives by making ammonia (basic) from urea using urease). 2. to provide the optimal pH for pepsin action 3. to activate pepsinogens (cleaved to form pepsin) 4. Facilitating absorption of iron by converting colloidal iron into ionic form. 5. stimulating duodenum to liberate secretin 6. breaks down connective tissue in food Prof. Dr.H.D.El-Yassin 12 2011 LECTURE 2 Thursday 29/9/2011 2.2.Mechanism of gastric acid secretion The hydrogen ion concentration in parietal cell secretions is roughly 3 million fold higher than in blood, HC1 at a concentration of roughly 160 mM (equivalent to a pH of 0.8). And chloride is secreted against both a concentration and electric gradient. Thus, the ability of the parietal cell to secrete acid is dependent on active transport. Acid secretion mechanisms in the parietal cell The key player in acid secretion is a H+/K+ ATPase or "proton pump" located in the cannalicular membrane. This ATPase is magnesiumdependent. Prof. Dr.H.D.El-Yassin 13 2011 LECTURE 2 Thursday 29/9/2011 The H+/K+ ATPase The parietal cells in the stomach use this pump to secrete gastric juice. These cells transport protons (H+) from a concentration of about 4 x 10-8 M within the cell to a concentration of about 0.15 M in the gastric juice (giving it a pH close to 1). Small wonder that parietal cells are stuffed with mitochondria and uses huge amounts of energy as they carry out this three-million fold concentration of protons. The current model for explaining acid secretion is as follows: • Hydrogen ions are generated within the parietal cell from dissociation of water. The hydroxyl ions formed in this process rapidly combine with carbon dioxide to form bicarbonate ion, a reaction cataylzed by carbonic anhydrase. • Bicarbonate is transported out of the basolateral membrane in exchange for chloride. The outflow of bicarbonate into blood results in a slight elevation of blood pH known as the "alkaline tide". This process serves to maintain intracellular pH in the parietal cell. • Chloride and potassium ions are transported into the lumen of the cannaliculus by conductance channels, and such is necessary for secretion of acid. • Hydrogen ion is pumped out of the cell, into the lumen, in exchange for potassium through the action of the proton pump; potassium is thus effectively recycled. Accumulation of osmotically-active hydrogen ion in the cannaliculus generates an osmotic gradient across the membrane that results in outward diffusion of water - the resulting gastric juice is 155 mM HC1 and 15 mM KC1 with a small amount of NaCl. Prof. Dr.H.D.El-Yassin 14 2011 LECTURE 2 Thursday 29/9/2011 2.3. Control of gastric acid secretion Parietal cells bear receptors for three stimulators of acid secretion, reflecting a neural, paracrine and endocrine control: ACETYLCHOLINE o o o o released from cholinergic nerve fibres binds to (M3) receptor on cell surface opens Ca++ channels in apical surface promotes release of Ca++ from intracellular stores GASTRIN o binds to CCK-B receptor on cell surface o releases intracellular Ca++ HISTAMINE o released from mast cells o binds to parietal cell surface receptor o activates adenyl cyclase (increases cyclic AMP, an intracellular messenger) Prof. Dr.H.D.El-Yassin 15 2011 LECTURE 2 Thursday 29/9/2011 Histamine's effect on the parietal cell is to activate adenylate cyclase, leading to elevation of intracellular cyclic AMP concentrations and activation of protein kinase A (PKA). One effect of PKA activation is phosphorylation of cytoskeletal proteins involved in transport of the H+/K+ ATPase from cytoplasm to plasma membrane. Binding of acetylcholine and gastrin both result in elevation of intracellular calcium concentrations. INHIBITORY CONTROL • acid at less than pH 2 is a direct inhibitor of acid release • acid in duodenum releases secretin which inhibits gastric secretion • fatty acids, peptides stimulate release of GIF (gastric inhibitory polypeptide) and CCK (cholecystokinin) Several additional mediators have been shown to result in gastric acid secretion when infused into animals and people, including e.g. calcium. Calcium simulates gastrin release. lt is unclear whether these molecules have a significant physiologic role in parietal cell function. Alkaline tide during gastric secretion: Owing to secreation of a lage amount of H+ as HCl, there is surplus of OH- in the parietal cell which is taken up not only by the CO2 to form HCO3- but also by other buffer systems of parietal cell initially and later by those of plasma. HPO 42 H 2 PO4 HCO3 H 2 CO3 Lactate Lactic acid All tend to increase on the side of the base i.e.:HPO4-2, HCO3and lactate, with the result that the pH of plasma is raised and an alkaline urine is excreted for some hours following intake of food and gastric secretion. This is known as the alkaline tide. Prof. Dr.H.D.El-Yassin 16 2011 LECTURE 2 Thursday 29/9/2011 3. Proteases: Pepsinogen, an inactive zymogen, is secreted into gastric juice from both mucous cells and chief cells. Once secreted, pepsinogen is activated by stomach acid into the active protease pepsin, which is largely responsible for the stomach's ability to initiate digestion of proteins, in young animals; chief cells also secrete chymosin (rennin), a protease that coagulates milk protein allowing it to be retained more than briefly in the stomach. Pepsinogens and Pepsins Pepsinogens are secreted in a form such that the activation peptide assumes a compact structure that occludes the active site. On exposure to an acidic (pH < 4) environment such as occurs in the lumen of the stomach, the activation peptide unfolds, allowing the active site to clip it off, yielding mature, catalytically active pepsin. Optimal activity of pepsins is at pH of 1.8 to 3.5, depending on the isoform, They are reversibly inactivated at about pH 5 and irreversibly inactivated at pH 7 to 8. The mature, active enzymes are roughly 325 amino acids with a mass of approximately 35 kDa. Pepsin initiates protein digestion by splitting certain amino acid linkages in proteins (Cleaves preferentially C-terminal. It does not cleave at V, A or G. Other residues may be cleaved, with very variable rates) to yield peptide fragments. 17 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 2 Thursday 29/9/2011 Because pepsin can digest protein, it must be stored and secreted in an inactive form so that it does not digest the cells in which it is formed. In general, secretion of pepsinogens is coupled to secretion of acid from the parietal cell. In vitro studies have demonstrated that secretion is effectively stimulated by agents that stimulate either of two conditions: 1. Elevated intracellular levels of cyclic AMP: examples include secretin, vasoactive intestinal peptide and epinephrine. 2. Elevated intracellular calcium: the principal mediators investigated include acetylcholine and peptides of the gastrin/cholecystokinin family Pepsin was discovered by Theodor Schwann in 1836. It was the first animal enzyme to be discovered. Chymosin (Rennin) and the Coagulation of Milk Chymosin, known also as rennin, is a proteolytic enzyme synthesized by chief cells in the stomach. Its role in digestion is to coagulate milk in the stomach, a process of considerable importance in the very young animal. If milk were not coagulated, it would rapidly flow through the stomach and miss the opportunity for initial digestion of its proteins. Chymosin efficiently converts liquid milk to a semisolid like cottage cheese, allowing it to be retained for longer periods in the stomach. Chymosin secretion is maximal during the first few days after birth, and declines thereafter, replaced in effect by secretion of pepsin as the major gastric protease. 18 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 2 Thursday 29/9/2011 Chymosin is secreted as an inactive proenzyme called prochymosin that, like pepsin, is activated on exposure to acid. Chymosin is also similar to pepsin in being most active in acidic environments, which makes sense considering its mission. Aside from its physiologic role, chymosin is also a very important industrial enzyme because it is widely used in cheese making. 4. Hormones The principle hormone secreted from the gastric epithelium is gastrin, a peptide that is important in control of acid secretion and gastric motility. Gastrin is secreted by G-cells and released into the blood where it travels to the parietal cells to stimulate acid secretion, and to Enterochromaffin-Like (ECL) Cells to stimulate histamine secretion. The net result of gastrin secretion is increased acid production through two mechanisms: 1. Direct stimulation of the parietal cells, 2. Tropic action on parietal cells increasing their number. N.B. in gastrinoma (Zollinger-Ellison syndrome) increased production of gastrin causes hypersecretion of acid which is not subject to normal inhibitory mechanisms. A number of other enzymes are secreted by gastric epithelial cells, including a lipase and gelatinase. One secretory product of considerable importance in man is intrinsic factor, a glycoprotein secreted by parietal cells that is necessary for intestinal absorption of vitamin B12. 19 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 2 Thursday 29/9/2011 Intrinsic Factor Intrinsic factor is a glycoprotein secreted by parietal (humans) of the gastric mucosa. In humans, it has an important role in the absorption of vitamin B12 (cobalamin) in the intestine, and failure to produce or utilize intrinsic factor results in the condition pernicious anemia.— as a result of an autoimmune attack against parietal cells — Dietary vitamin B12 is released from ingested proteins in the stomach through the action of pepsin and acid. It is rapidly bound by one of two vitamin B12-binding proteins that are present in gastric juice; at acid pH, these binding proteins have a greater affinity for the vitamin than does intrinsic factor. In the small intestine pancreatic proteases digest the binding proteins, releasing vitamin B12 which then becomes bound to intrinsic factor. Finally, there are receptors for intrinsic factor on the ileal mucosa which bind the complex, allowing vitamin B12 to be absorbed into portal blood. In all mammals, vitamin B12is necessary for maturation of erythrocytes, and a deficiency of this vitamin leads to development of anemia. Since efficient absorption of vitamin B12in humans depends on intrinsic factor, diseases which decrease the secretion of intrinsic factor (e.g. atrophic gastritis), interfere with cleavage of the binding proteins (e.g. pancreatic exocrine insufficiency) or decrease binding and absorption of the intrinsic factor-vitamin B12 complex (e.g. ileal disease or resection) can result in this type of anemia. Absorption in the Stomach The stomach absorbs very few substances, although small amounts of certain lipid-soluble compounds can be taken up, including aspirin, other non-steroidal anti-inflammatory drugs, and ethanol. Notably, these substances are also well-recognized causes of gastric irritation and their use (especially overuse) is commonly associated with development of gastritis and gastric ulcers. 20 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 Digestive Secretion from Pancreas and Liver The Pancreas The pancreas plays a vital role in accomplishing the followings: • Acid must be quickly and efficiently neutralized to prevent damage to the duodenal mucosa • Macromolecular nutrients - proteins, fats and starch - must be broken down much further before their constituents can be absorbed through the mucosa into blood Insufficient exocrine secretion by the pancreas leads to starvation, even if the body is consuming adequate quantities of high quality food. In addition to its role as an exocrine organ, the pancreas is also an endocrine organ. The major hormones it secretes - insulin and glucagon - play a vital role in carbohydrate and lipid metabolism. Exocrine Secretions of the Pancreas 21 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 Pancreatic juice is composed of two secretory products critical to proper digestion: digestive enzymes and bicarbonate. The enzymes are synthesized and secreted from the exocrine acinar cells, whereas bicarbonate is secreted from the epithelial cells lining small pancreatic ducts. 1. Digestive Enzymes: a. Proteases Digestion of proteins is initiated by pepsin in the stomach, but the bulk of protein digestion is due to the pancreatic proteases. Several proteases are synthesized in the pancreas and secreted into the lumen of the small intestine. The two major pancreatic proteases are trypsin and chymotrypsin both are endopeptidases, which are synthesized and packaged into secretory vesicles as the inactive proenzymes trypsinogen and chymotrypsinogen. Trypsin: Cleaves peptide bonds on the C-terminal side of arginines and lysines. Chymotrypsin: Cuts on the C-terminal side of tyrosine, phenylalanine, and tryptophan residues (the same bonds as pepsin, whose action ceases when the NaHCOs raises the pH of the intestinal contents). 22 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 Once trypsinogen and chymotrypsinogen are released into the lumen of the small intestine, they must be converted into their active forms in order to digest proteins, Trypsinogen is activated by the enzyme enterokinase, which is embedded in the intestinal mucosa. Once trypsin is formed, it activates chymotrypsinogen, as well as additional molecules of trypsinogen. The net result is a rather explosive appearance of active protease once the pancreatic secretions reach the small intestine. Trypsin and chymotrypsin digest proteins into peptides and peptides into smaller peptides, but they cannot digest proteins and peptides to single amino acids. Some of the other proteases from the pancreas, for instance carboxypeptidase (exopeptidase) (This enzyme removes, one by one, the amino acids at the C-terminal of peptides). But the final digestion of peptides into amino acids is largely the effect of peptidases in small intestinal epithelial cells. 23 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 b. Pancreatic Lipase The major form of dietary fat is triglyceride, or neutral lipid. A triglyceride molecule cannot be directly absorbed across the intestinal mucosa. It must first be digested into a 2-monoglyceride and two free fatty acids. The enzyme that performs this hydrolysis is pancreatic lipase. Sufficient quantities of bile salts must also be present in the lumen of the intestine in order for lipase to efficiently digest dietary triglyceride and for the resulting fatty acids and monoglyceride to be absorbed. This means that normal digestion and absorption of dietary fat is critically dependent on secretions from both the pancreas and liver. Pancreatic lipase has recently been in the limelight as a target for management of obesity. The drug orlistat (Xenical) is a pancreatic lipase inhibitor that interferes with digestion of triglyceride and thereby reduces absorption of dietary fat. Clinical trials support the contention that inhibiting lipase can lead to significant reductions in body weight in some patients. c. Amylase The major dietary carbohydrate for many species is starch, a storage form of glucose in plants. Amylase is the enzyme that hydrolyses starch to maltose (a glucose-glucose disaccharide), as well as the trisaccharide maltotriose and small branchpoints fragments called dextrins. d. Other Pancreatic Enzymes In addition to the proteases, lipase and amylase, the pancreas produces a host of other digestive enzymes, including nucleases, gelatinase and elastase. Nucleases. These hydrolyze ingested nucleic acids (RNA and DNA) into their component nucleotides. Elastase: Cuts peptide bonds next to small, uncharged side chains such as those of alanine and serine. 24 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 2. Bicarbonate and Water Epithelial cells in pancreatic ducts are the source of the bicarbonate and water secreted by the pancreas. The mechanism of bicarbonate secretion is essentially the same as for acid secretion parietal cells and is dependent on the enzyme carbonic anhydrase. In pancreatic duct cells, the bicarbonate is secreted into the lumen of the duct and hence into pancreatic juice. Control of Pancreatic Exocrine Secretion Secretion from the exocrine pancreas is regulated by both neural and endocrine controls. During interdigestive periods, very little secretion takes place, but as food enters the stomach and, a little later, chyme flows into the small intestine, pancreatic secretion is strongly stimulated. 25 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 The most important stimuli for pancreatic secretion come from three hormones secreted by the enteric endocrine system: • Cholecystokinin: This hormone is synthesized and secreted by enteric endocrine cells located in the duodenum. Its secretion is strongly stimulated by the presence of partially digested proteins and fats in the small intestine. As chyme floods into the small intestine, cholecystokinin is released into blood and binds to receptors on pancreatic acinar cells, ordering them to secrete large quantities of digestive enzymes. It also stimulates the gallbladder to release bile and the pancreas to produce pancreatic enzymes. • Secretin: This hormone is secreted in response to acid in the duodenum. The predominant effect of secretin on the pancreas is to stimulate duct cells to secrete water and bicarbonate. As soon as this occurs, the enzymes secreted by the acinar cells are flushed out of the pancreas, through the pancreatic duct into the duodenum. It also stimulates the liver to secrete bile. • Gastrin: This hormone, which is very similar to cholecystokinin, is secreted in large amounts by the stomach in response to gastric distention and irritation, in addition to stimulating acid secretion by the parietal cell; gastrin stimulates pancreatic acinar cells to secrete digestive enzymes. 26 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 The Liver The liver is the largest gland in the body and performs an astonishingly large number of tasks that impact all body systems. One consequence of this complexity is that hepatic disease has widespread effects on virtually all other organ systems. The three fundamental roles of the liver are: 1. Vascular functions: including formation of lymph and hepatic phagocytic system. 2. Metabolic achievements in control of synthesis and utilization of carbohydrates, lipids and proteins. 3. Secretory and excretory functions, particularly with respect to the synthesis of secretion of bile. The latter is the only one of the three that directly affects digestion - the liver, through its biliary tract, secretes bile acids into the small intestine where they assume a critical role in the digestion and absorption of dietary lipids. 27 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 Secretion of Bile and the Role of Bile Acids in Digestion Bile is a complex fluid containing water, electrolytes and a battery of organic molecules including bile acids, cholesterol, phospholipids and bilirubin that flows through the biliary tract into the small intestine. There are two fundamentally important functions of bile in all species: Bile contains bile acids, which are critical for digestion and absorption of fats and fat-soluble vitamins in the small intestine. Many waste products are eliminated from the body by secretion into bile and elimination in feces. The secretion of bile can be considered to occur in two stages: • Initially, hepatocytes secrete bile into canaliculi, from which it flows into bile ducts. This hepatic bile contains large quantities of bile acids, cholesterol and other organic molecules. • As bile flows through the bile ducts it is modified by addition of a watery, bicarbonate-rich secretion from ductal epithelial cells. In humans: the gall bladder stores and concentrates bile during the fasting state. Typically, bile is concentrated five-fold in the gall bladder by absorption of water and small electrolytes - virtually all of the organic molecules are retained. Secretion into bile is a major route for eliminating cholesterol. Free cholesterol is virtually insoluble in aqueous solutions, but in bile, it is made soluble by bile acids and lipids like lethicin. Gallstones (Cholelithiasis) most of which are composed predominantly of cholesterol, result from processes that allow cholesterol to precipitate from solution in bile. 28 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 Role of Bile Acids in Fat Digestion and Absorption Bile salts are formed in the hepatocytes by a series of enzymatic steps that convert cholesterol to cholic or chenodeoxycholic acids. The rate limiting step is hydroxylation at the 7-alpha position. These reactions include the activity of 8 enzymes belonging to either monooxygenase or dehydrogenase enzyme classes. There are four major bile acids found in the body: 1. Cholic acid 2. Deoxycholic acid 3. Decholin 4. Chenodiol (Chenix), is used to dissolve gallstones in patients who cannot tolerate surgery. Chenodiol is a natural bile acid that blocks production of cholesterol . This action leads to gradual dissolution of cholesterol gallstones. 29 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 Synthesis of bile acids is one of the predominant mechanisms for the excretion of excess cholesterol. However, the excretion of cholesterol in the form of bile acids is insufficient to compensate for an excess dietary intake of cholesterol. These acids are then conjugated with glycine or taurine and secreted as Na+ (or K+) salts. Conjugation causes a decrease in their pKa values, making them more water soluble. The most abundant bile acids in human bile are chenodeoxycholic acid (45%) and cholic acid (31%). These are referred to as the primary bile acids. Within the intestines the primary bile acids are acted upon by bacteria and converted to the secondary bile acids, identified as deoxycholate (from cholate) and lithocholate (from chenodeoxycholate). Both primary and secondary bile acids are reabsorbed by the intestines and delivered back to the liver via the portal circulation. Within the liver the carboxyl group of primary and secondary bile acids is conjugated via an amide bond to either glycine or taurine before their being resecreted into the bile canaliculi. These conjugation reactions yield glycoconjugates and tauroconjugates, respectively. The bile canaliculi join with the bile ductless, which then form the bile ducts. Bile acids are carried from the liver through these ducts to the gallbladder, where they are stored for future use. The ultimate fate of bile acids is secretion into the intestine, where they aid in the emulsification of dietary lipids. In the gut the glycine and taurine residues are removed and the bile acids are either excreted (only a small percentage) or reabsorbed by the gut and returned to the liver. This process of secretion from the liver to the gallbladder, to the intestines and finally reabsorbtion is termed the enterohepatic circulation. 30 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 Enterohepatic Recirculation After the bile acids has been released into the small intestine via the bile duct to play an integral role in the absorption of dietary lipids and lipid soluble vitamins. More than 90% of the bile salts are actively reabsorbed (by a sodium-dependent cotransport process) from the ileum into the hepatic-portal circulation from where they are cleared and resecreted by the liver to once again be stored in the gall bladder. This secretion/reabsorption cycle is called the Enterohepatic Circulation. Systemic circulation: supplies nourishment to all of the tissue located throughout your body, with the exception of the heart and lungs because they have their own systems. Systemic circulation is a major part of the overall circulatory system. Portal circulation: Blood from the gut and spleen flow to and through the liver before returning to the right side of the heart. This is called the portal circulation and the large vein through which blood is brought to the liver is called the portal vein. The net effect of this enterohepatic recirculation is that each bile salt molecule is reused about 20 times, often two or three times during a single digestive phase. 31 Prof. Dr. Hedef Dhafir El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 Note: liver disease can dramatically alter this pattern of recirculation - for instance, sick hepatocytes have decreased ability to extract bile acids from portal blood and damage to the canalicular system can result in escape of bile acids into the systemic circulation. Assay of systemic levels of bile acids is used clinically as a sensitive indicator of hepatic disease. Bile acids are facial amphipathic, that is, they contain both hydrophobic (lipid soluble) and polar (hydrophilic) faces. The cholesterol-derived portion of a bile acid has one face that is hydrophobic (that with methyl groups) and one that is hydrophilic (that with the hydroxyl groups); the amino acid conjugate is polar and hydrophilic. Their amphipathic nature enables bile acids to carry out two important functions: 1. Emulsification of lipid aggregates: Bile acids have detergent action on particles of dietary fat, which causes fat globules to break down or be emulsified into minute, microscopic droplets. Emulsification is not digestion per se, but is of importance because it greatly increases the surface area of fat, making it available for digestion by lipases, which cannot access the inside of lipid droplets. 2. Solubilization and transport of lipids in an aqueous environment: Bile acids are lipid carriers and are able to solubilize many lipids by forming micelles - aggregates of lipids such as fatty acids, cholesterol and monoglycerides - that remain suspended in water. Bile acids are also critical for transport and absorption of the fat-soluble vitamins. Solubility properties of bile acids in aqueous solutions. Abbreviation: CMC, critical micellar concentration 32 Prof.Dr H.D.El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 Clinical Significance of Bile Acid Synthesis Bile acids perform four physiologically significant functions: 1. Their synthesis and subsequent excretion in the feces represent the only significant mechanism for the elimination of excess cholesterol. 2. Bile acids and phospholipids solubilize cholesterol in the bile, thereby preventing the precipitation of cholesterol in the gallbladder. 3. They facilitate the digestion of dietary triacylglycerols by acting as emulsifying agents that render fats accessible to pancreatic lipases. 4. They facilitate the intestinal absorption of fat-soluble vitamins. Role of Bile Acids in Cholesterol Homeostasis Hepatic synthesis of bile acids accounts for the majority of cholesterol breakdown in the body. In humans, roughly 500 mg of cholesterol are converted to bile acids and eliminated in bile every day. This route for elimination of excess cholesterol is probably important in all animals, but particularly in situations of massive cholesterol ingestion. Interestingly, it has recently been demonstrated that bile acids participate in cholesterol metabolism by functioning as hormones that alter the transcription of the rate-limiting enzyme in cholesterol biosynthesis. Pattern and Control of Bile Secretion The flow of bile is lowest during fasting, and a majority of that is diverted into the gallbladder for concentration. When chyme from an ingested meal enters the small intestine, acid and partially digested fats and proteins stimulate secretion of cholecystokinin and secretin. These enteric hormones have important effects on pancreatic exocrine secretion. They are both also important for secretion and flow of bile: 33 Prof.Dr H.D.El-Yassin 2011 LECTURE 3 Sunday 2/10/2011 1. Cholecystokinin: The name of this hormone describes its effect on the biliary system - cholecysto = gallbladder and kinin = movement. The most potent stimulus for release of cholecystokinin is the presence of fat in the duodenum. Once released, it stimulates contractions of the gallbladder and common bile duct, resulting in delivery of bile into the gut. 2. Secretin: This hormone is secreted in response to acid in the duodenum. Its effect on the biliary system is very similar to what was seen in the pancreas - it simulates biliary duct cells to secrete bicarbonate and water, which expands the volume of bile and increases its flow out into the intestine. 34 Prof.Dr H.D.El-Yassin 2011 LECTURE 4 Tuesday 4/10/2011 Detoxification in the Liver The liver is one of the most important organs in the body when it comes to detoxifying or getting rid of foreign substances or toxins, especially from the gut. The liver detoxifies harmful substances by a complex series of chemical reactions. The role of these various enzyme activities in the liver is to convert fat soluble toxins into water soluble substances that can be excreted in the urine or the bile depending on the particular characteristics of the end product. Many of the toxic chemicals that enter the body are fatsoluble, which means they dissolve only in fatty or oily solutions and not in water. This makes them difficult for the body to excrete. Fat soluble chemicals have a high affinity for fat tissues and cell membranes, which are composed of fatty acids and proteins. In these fatty tissues of the body, toxins may be stored for years, being released during times of exercise, stress or fasting. The liver plays several roles in detoxification: it filters the blood to remove large toxins, synthesizes and secretes bile full of cholesterol and other fatsoluble toxins, and enzymatically disassembles unwanted chemicals. LECTURE 4 Tuesday 4/10/2011 35 Prof.Dr H.D.El-Yassin 2011 LECTURE 4 Tuesday 4/10/2011 This enzymatic process usually occurs in two steps referred to as: phase I and phase II. Phase I either directly neutralizes a toxin, or modifies the toxic chemical to form activated intermediates which are then neutralized by one of more of the several phase II enzyme systems. The level of exposure to environmental carcinogens varies widely, as does the efficiency of the detoxification enzymes, particularly phase II. High levels of exposure to carcinogens coupled with slow detoxification enzymes significantly increases susceptibility to cancer. 36 Prof.Dr H.D.El-Yassin 2011 LECTURE 4 Tuesday 4/10/2011 Phase I Detoxification This pathway converts a toxic chemical into a less harmful chemical. This is achieved by various chemical reactions (such as oxidation, reduction and hydrolysis), and during this process free radicals are produced which, if excessive, can damage the liver cells. Antioxidants reduce the damage caused by these free radicals. If antioxidants are lacking and toxin exposure is high, toxic chemicals become far more dangerous. Some may be converted from relatively harmless substances into potentially carcinogenic substances. The effects of exposure to toxins varies from individual to individual. Some people are highly sensitive to different endogenous and exogenous toxins. Others, because their bodies are more resilient and their livers can detoxify more efficiently, aren't as sensitive. CYTOCHROME P450 MONOOXYGENASE SYSTEM Monooxygenase (mixed function oxidases) incorporate one atom from molecular oxygen into a substrate (creating a hydroxyl group), with the other atom being reduced to water. In the cytochrome P450 monooxygenase system NADPH provides the reducing equivalents required by the series of reactions. This system performs different functions in two separate locations in cells.The overall reaction catalyzed by a cytochrome P450 enzyme is: R-H + O2 + NADPH + H+ R-OH + H2O + NADP+ where R may be a steroid, drug or other chemical. 1. Mitochondrial System: the function of the mitochondrial cytochrome P450 monooxygenase system is to participate in the hydroxylation of steroids, a process that makes theses hydrophobic compounds more water soluble. For example, in the steroid hormone producing tissues, such as placenta, ovaries, testes and adrenal cortex, it is used to hydroxylate intermediates in the conversion of cholesterol to steroid hormones. The liver uses this system in bile acid synthesis, and kidney uses it to hydroxylat vitamin 25-hydroxycholecalciferol (vitamin D) to its biologically active 1, 25-hydroxylated form. 37 Prof.Dr H.D.El-Yassin 2011 LECTURE 4 Tuesday 4/10/2011 2. Microsomal System: An extremely important function the function of the microsomal cytochrome P450 monooxygenase system found associated with the membranes of the endoplasmic reticulum (particularly in the liver) is the detoxification of foreign compounds (xenobiotics). Theses include numerous drugs such as varied pollutants as petroleum products, carcinogens and pesticides. The cytochrome P450 monooxygenase system can be used to hydroxylate theses toxins again using NADPH as the source of reducing equivalents. The purpose of these modifications is: a. it may itself activate or deactivate a drug, b. or make a toxic compound more soluble, thus facilitating its excretion in the urine or feces. Frequently, however, the new hydroxyl group will serve as a site for conjugation with polar compound, such as glucuronic acid, which will significantly increase the compound's solubility. Excessive amounts of toxic chemicals such as pesticides can disrupt the P450 enzyme system by causing hyper activity or what is called 'induction' of this pathway. This will result in high levels of damaging free radicals being produced. Substances that may cause hyperactivity of the P- 450 enzymes: Caffeine, Alcohol, Dioxin, Saturated fats, Organophosphorus pesticides, Paint fumes, Sulfonamides, Exhaust fumes, Barbiturates. Transforming a toxin to a more chemically reactive form makes it more easily metabolized by the phase II enzymes. If the phase II detoxification systems are not working adequately, these intermediates can cause substantial damage, including the initiation of carcinogenic processes. Each enzyme works best in detoxifying certain types of chemicals, but with considerable overlap in activity among the enzymes. The activity of the various cytochrome P450 enzymes varies significantly from one individual to another, based on genetics, the individual's level of exposure to chemical toxins, and his or her nutritional status. Since the activity of cytochrome P450 varies so much, so does an individual's risk for various diseases. This variability of cytochrome P450 enzymes is seen in the variability of people's ability to detoxify the carcinogens found in cigarette smoke and helps to explain why some people can smoke with only modest damage to their lungs, while others develop lung cancer after only a few decades of smoking. LECTURE 4 Tuesday 4/10/2011 38 Prof.Dr H.D.El-Yassin 2011 LECTURE 4 Tuesday 4/10/2011 A significant side-effect of phase I detoxification is the production of free radicals as the toxins are transformed--for each molecule of toxin metabolized by phase I, one molecule of free radical is generated. Without adequate free radical defenses, every time the liver neutralizes a toxin exposure, it is damaged by the free radicals produced. The most important antioxidant for neutralizing the free radicals produced in phase I is glutathione. In the process of neutralizing free radicals, however, glutathione (GSH) is oxidized to glutathione disulfide (GSSG). Glutathione is required for one of the key phase II detoxification processes. When high levels of toxin exposure produce so many free radicals from phase I detoxification that the glutathione is depleted, the phase II processes dependent upon glutathione stop, producing oxidative stress or liver damage. The toxins transformed into activated intermediates by phase I are substantially more reactive than the phase I toxins were. Unless quickly removed from the body by phase II detoxification mechanisms, they can cause widespread problems, especially carcinogenesis. Therefore, the rate at which phase I produces activated intermediates must be balanced by the rate at which phase II finishes their processing. People with a very active phase I detoxification system coupled with slow or inactive phase II enzymes are termed pathological detoxifiers. These people suffer unusually severe toxic reactions to environmental poisons. An efficient liver detoxification system is vital to health and in order to support this process it is essential that many key nutrients are included in the diet. Vitamins and minerals – particularly the B vitamins – play a major role, acting as cofactors for many enzyme systems including those of liver detoxification. Depletion of vitamin C may also impair the detoxification process; vitamin C also prevents free radical formation. Vitamin E and selenium are cofactors for glutathione peroxidase activity as well as being powerful antioxidants. Other nutrients which play vital roles in the Phase II pathway include amino acids glycine, cysteine, glutamine, methionine, taurine, glutamic acid and aspartic acid. Grapefruit juice, which contains naringenin, slows down Phase I enzyme activity. As with all enzymes, the cytochrome P450s require several nutrients to function, such as copper, magnesium, zinc and vitamin C. 39 Prof.Dr H.D.El-Yassin 2011 LECTURE 4 Tuesday 4/10/2011 Phase II Detoxification This is called the conjugation pathway, whereby the liver cells add another substance (eg. cysteine, glycine or a sulphur molecule) to a toxic chemical or drug. This makes the toxin or drug water-soluble, so it can then be excreted from the body via watery fluids such as bile or urine. Individual xenobiotics and metabolites usually follow one or two distinct pathways. There are essentially six phase II detoxification pathways: 1. 2. 3. 4. 5. 6. Glutathione conjugation Amino acid conjugation Methylation Sulfation Acetylation Glucuronidation 1. Glutathione conjugation A primary phase II detoxification route is conjugation with glutathione(glutamylcysteinylglycine), (a tripeptide composed of three amino acids--cysteine, glutamic acid, and glycine). Glutathione conjugation produces watersoluble mercaptates which are excreted via the kidneys. The elimination of fat-soluble compounds, especially heavy metals like mercury and lead, is dependent upon adequate levels of glutathione, which in turn is dependent upon adequate levels of methionine and cysteine. When increased levels of toxic compounds are present, more methionine is utilized for cysteine and glutathione synthesis. Methionine and cysteine have a protective effect on glutathione and prevent depletion during toxic overload. This, in turn, protects the liver from the damaging effects of toxic compounds and promotes their elimination. If the availability of methionine is reduced, not only will the capability of the liver to detoxify be impaired, but there will also be less glutathione available to complex with foreign substances. Studies have demonstrated that a deficiency of methionine can, in itself, cause liver cancer without the presence of a carcinogen, and also that the deficiency of methionine can permit a heavy metal to cause toxic effects. LECTURE 4 Tuesday 4/10/2011 40 Prof.Dr H.D.El-Yassin 2011 LECTURE 4 Tuesday 4/10/2011 Glutathione is also an important antioxidant. This combination of detoxification and free radical protection, results in glutathione being one of the most important anticarcinogens and antioxidants in our cells, which means that a deficiency is cause of serious liver dysfunction and damage. Exposure to high levels of toxins depletes glutathione faster than it can be produced or absorbed from the diet. This results in increased susceptibility to toxin-induced diseases, such as cancer, especially if phase I detoxification system is highly active. A deficiency can be induced either by diseases that increase the need for glutathione, deficiencies of the nutrients needed for synthesis, or diseases that inhibit its formation. Glutathione is available through two routes: diet and synthesis. Dietary glutathione (found in fresh fruits and vegetables, cooked fish, and meat) is absorbed well by the intestines and does not appear to be affected by the digestive processes. Dietary glutathione in foods appears to be efficiently absorbed into the blood. 2. Amino acid conjugation Several amino acids (glyucine, taurine, glutamine, arginine, and ornithine) are used to combine with and neutralize toxins. Of these, glycine is the most commonly utilized in phase II amino acid detoxification. Patients suffering from hepatitis, alcoholic liver disorders, carcinomas, chronic arthritis, hypothyroidism, toxemia of pregnancy, and excessive chemical exposure are commonly found to have a poorly functioning amino acid conjugation system. Even in normal adults, a wide variation exists in the activity of the glycine conjugation pathway. This is due not only to genetic variation, but also to the availability of glycine in the liver. Glycine, and the other amino acids used for conjugation, become deficient on a low-protein diet and when chronic exposure to toxins results in depletion. 3. Methylation Methylation involves conjugating methyl groups to toxins. Most of the methyl groups used for detoxification comes from Sadenosylmethionine (SAM). SAM is synthesized from the amino acid methionine, a process which requires the nutrients choline, the active form of B12 --methyl cobalamin, and the active form of folic acid --5methyltetrahydrofolate. Methionine is a major source of numerous sulfurcontaining compounds, including the amino acids cysteine and taurine. 41 Prof.Dr H.D.El-Yassin 2011 LECTURE 4 Tuesday 4/10/2011 4. Sulfation Sulfation is the conjugation of toxins with sulfur-containing compounds. The sulfation system is important for detoxifying several drugs, food additives, and, especially, toxins from intestinal bacteria and the environment. In addition to environmental toxins, sulfation is also used to detoxify some normal body chemicals and is the main pathway for the elimination of steroid and thyroid hormones. Since sulfation is also the primary route for the elimination of neurotransmitters, dysfunction in this system may contribute to the development of some nervous system disorders. Many factors influence the activity of sulfate conjugation. For example, a diet low in methionine and cysteine has been shown to reduce sulfation. 5. Acetylation Conjugation of toxins with acetyl-CoA is the primary method by which the body eliminates sulfa drugs. This system appears to be especially sensitive to genetic variation, with those having a poor acetylation system being far more susceptible to sulfa drugs and other antibiotics. While not much is known about how to directly improve the activity of this system, it is known that acetylation is dependent on thiamine, pantothenic acid, and vitamin C. 6. Glucuronidation Glucuronidation, the combining of glucuronic acid with toxins, in Phase II can be reversed by Beta glucuronidase enzymes produced by pathological bacteria and cause toxins to be reabsorbed increasing toxicity. Many of the commonly prescribed drugs are detoxified through this pathway. It also helps to detoxify aspirin, menthol, vanillin (synthetic vanilla), food additives such as benzoates, and some hormones. Sulfoxidation Sulfoxidation is the process by which the sulfur-containing molecules in drugs and foods are metabolized. It is also the process by which the body eliminates the sulfite food additives used to preserve many foods and drugs. Normally, the enzyme sulfite oxidase (molybdenum dependentenzyme) metabolizes sulfites to safer sulfates, which are then excreted in the urine. Those with a poorly functioning sulfoxidation system, however, have an increased ratio of sulfite to sulfate in their urine. Those with a poorly functioning sulfoxidation detoxification pathway are more sensitive to sulfur-containing drugs and foods containing sulfur or sulfite additives. Lecture 5 Thursday 6/10/2011 42 Prof.Dr H.D.El-Yassin 2011 Lecture 5 Thursday 6/10/2011 SECRETION AND ABSORPTION IN THE SMALL INTESTINE The small intestine is the portal for absorption of virtually all nutrients into blood. Accomplishing this transport requires breaking down large supramolecular aggregates into small molecules that can be transported across the epithelium. By the time ingesta reaches the small intestine, foodstuffs have been mechanically broken down and reduced to a liquid by mastication and grinding in the stomach. Once within the small intestine, these macromolecular aggregates are exposed to pancreatic enzymes and bile, which enables digestion to molecules capable or almost capable of being absorbed. The final stages of digestion occur on the surface of the small intestinal epithelium. The net effect of passage through the small intestine is absorption of most of the water and electrolytes (sodium, chloride, potassium) and essentially all dietary organic molecules (including glucose, amino acids and fatty acids). Through these activities, the small intestine not only provides nutrients to the body, but plays a critical role in water and acid-base balance. Secretion in the Small Intestine Large quantities of water are secreted into the lumen of the small intestine during the digestive process. Almost all of this water is also reabsorbed in the small intestine. Regardless of whether it is being secreted or absorbed, water flows across the mucosa in response to osmotic gradients. In the case of secretion, two distinct processes establish an osmotic gradient that pulls water into the lumen of the intestine: 1. Increases in luminal osmotic pressure resulting from influx and digestion of foodstuffs: The chyme that floods into the intestine from the stomach typically is not hyperosmotic, but as its macromolecular components are digested, osmolarlity of that solution increases dramatically. Starch, for example, is a huge molecule that contributes only a small amount to osmotic pressure, but as it is digested, thousands of molecules of maltose are generated, each of which is as osmotically active as the original starch molecule. 43 Prof.Dr H.D.El-Yassin 2011 Lecture 5 Thursday 6/10/2011 Thus, as digestion proceeds lumenal osmolarity increases dramatically and water is pulled into the lumen. Then, as the osmotically active molecules (maltose, glucose, amino acids) are absorbed, osmolarity of the intestinal contents decreases and water can be absorbed. 2. Crypt cells actively secrete electrolytes, leading to water secretion: The apical or lumenal membrane of crypt epithelial cells contain an ion channel of immense medical significance - a cyclic AMP-dependent chloride channel known also as the cystic fibrosis transmembrane conductance regulator or CFTR. Mutations in the gene for this ion channel result in the disease cystic fibrosis. This channel is responsible for secretion of water by the following steps: 1. Chloride ions enter the crypt epithelial cell by cotransport with sodium and potassium; sodium is pumped back out via sodium pumps, and potassium is exported via a number of channels. 2. Activation of adenylyl cyclase by a number of so-called secretagogues leads to generation of cyclic AMP. 3. Elevated intracellular concentrations of cAMP in crypt cells activate the CFTR, resulting in secretion of chloride ions into the lumen. 4. Accumulation of negatively-charged chloride anions in the crypt creates an electric potential that attracts sodium, pulling it into the lumen, apparently across tight junctions - the net result is secretion of NaCl. 5. Secretion of NaCl into the crypt creates an osmotic gradient across the tight junction and water is drawn into the lumen. 44 Prof.Dr H.D.El-Yassin 2011 Lecture 5 Thursday 6/10/2011 CLINICAL CORRELATION Cystic fibrosis Abnormal activation of the cAMP-dependent chloride channel (CFTR) in crypt cells has resulted in the deaths of millions upon millions of people. Several types of bacteria produce toxins that strongly, often permanently, activate the adenylate cyclase in crypt enterocytes. This leads to elevated levels of cAMP, causing the chloride channels to essentially become stuck in the "open" position". The result is massive secretion of water that is manifest as severe diarrhea. Cholera toxin, produced by cholera bacteria, is the best known example of this phenomenon, but several other bacteria produce toxins that act similarly. Absorption in the Small Intestine: General Mechanisms Virtually all nutrients from the diet are absorbed into blood across the mucosa of the small intestine.To remain viable, all cells are required to maintain a low intracellular concentration of sodium. In polarized epithelial cells like enterocytes, low intracellular sodium is maintained by a large number of Na+/K+ ATPases - so-called sodium pumps - embedded in the basolateral membrane. These pumps export 3 sodium ions from the cell in exchange for 2 potassium ions, thus establishing a gradient of both charge and sodium concentration across the basolateral membrane. Aside from the electrochemical gradient of sodium, several other concepts are required to understand absorption in the small intestine. Also, dietary sources of protein, carbohydrate and fat must all undergo the final stages of chemical digestion just prior to absorption of, for example, amino acids, glucose and fatty acids. Water and electrolytes Carbohydrates, after digestion to monosaccharides Proteins, after digestion to small peptides and amino acids Neutral fat, after digestion to monoglyceride and free fatty acids 45 Prof.Dr H.D.El-Yassin 2011 Lecture 5 Thursday 6/10/2011 1) Absorption of Water and Electrolytes The small intestine must absorb massive quantities of water. A normal person akes in roughly 1 to 2 liters of dietary fluid every day. On top of that, another 6 to 7 liters of fluid is received by the small intestine daily as secretions from salivary glands, stomach, pancreas, liver and the small intestine itself. By the time the ingesta enters the large intestine, approximately 80% of this fluid has been absorbed. Net movement of water across cell membranes always occurs by osmosis, and the fundamental concept needed to understand absorption in the small gut is that absorption of water is absolutely dependent on absorption of solutes, particularly sodium: Sodium is absorbed into the cell by several mechanisms, but chief among them is by co-transport with glucose and amino acids - this means that efficient sodium absorption is dependent on absorption of these organic solutes. Absorbed sodium is rapidly exported from the cell via sodium pumps - when a lot of sodium is entering the cell, a lot of sodium is pumped out of the cell, which establishes a high osmolarity in the small intercellular spaces between adjacent enterocytes. Water diffuses in response to the osmotic gradient established by sodium - in this case into the intercellular space. It seems that the bulk of the water absorption is transcellular, but some also diffuses through the tight junctions. Water, as well as sodium, then diffuses into capillary blood within the villus. Water is thus absorbed into the intercellular space by diffusion down an osmotic gradient. However, looking at the process as a whole, transport of water from lumen to blood is often against an osmotic gradient - this is important because it means that the intestine can absorb water into blood even when the osmolarity in the lumen is higher than osmolarity of blood. 2) Absorption of Monosaccharides Monosaccharides, are only rarely found in normal diets. Rather, they are derived by enzymatic digestion of more complex carbohydrates within the digestive tube. Particularly important dietary carbohydrates include starch and disaccharides such as lactose and sucrose. None of these molecules can be absorbed for the simple reason that they cannot cross cell membranes unaided and, unlike the situation for monosaccharides, there are no transporters to carry them across. Brush Border Hydrolases Generate Monosaccharides Polysaccharides and disaccharides must be digested to monosaccharides prior to absorption and the key players in these processes are the brush border hydrolases, which include maltase, lactase and sucrase. Dietary lactose and sucrose are "ready" for digestion by their respective brush border enzymes. Starch, as discussed previously, is first digested to maltose by amylase in pancreatic secretions and, saliva. 46 Prof.Dr H.D.El-Yassin 2011 Lecture 5 Thursday 6/10/2011 Dietary lactose and sucrose, and maltose derived from digestion of starch, diffuse in the small intestinal lumen and come in contact with the surface of absorptive epithelial cells covering the villi where they engage with brush border hydrolases: maltase cleaves maltose into two molecules of glucose lactase cleaves lactose into a glucose and a galactose sucrase cleaves sucrose into a glucose and a fructose Glucose and galactose are taken into the enterocyte by cotransport with sodium using the same transporter. Fructose enters the cell from the intestinal lumen via facilitated diffusion through another transporter. Absorption of Glucose and other Monosaccharides: Transport across the Intestinal Epithelium Absorption of glucose entails transport from the intestinal lumen, across the epithelium and into blood. The transporter that carries glucose and galactose into the enterocyte is the sodium-dependent hexose transporter, known more formally as SGLUT-1. As the name indicates, this molecule transports both glucose and sodium ion into the cell and in fact, will not transport either alone. The essence of transport by the sodium-dependent hexose transporter involves a series of conformational changes induced by binding and release of sodium and glucose, and can be summarized as follows: 1. the transporter is initially oriented facing into the lumen - at this point it is capable of binding sodium, but not glucose 2. sodium binds, inducing a conformational change that opens the glucose-binding pocket 3. glucose binds and the transporter reorients in the membrane such that the pockets holding sodium and glucose are moved inside the cell 4. sodium dissociates into the cytoplasm, causing glucose binding to destabilize 5. glucose dissociates into the cytoplasm and the unloaded transporter reorients back to its original, outward-facing position Fructose is not co-transported with sodium. Rather it enters the enterocyte by another hexose transporter (GLUT5). Once inside the enterocyte, glucose and sodium must be exported from the cell into blood. Sodium is rapidly shuttled out in exchange for potassium by the battery of sodium pumps on the basolateral membrane, this process maintains the electrochemical gradient across the epithelium. The massive transport of sodium out of the cell establishes the osmotic gradient responsible for absorption of water. Digestion and absorption of carbohydrates 47 Prof.Dr H.D.El-Yassin 2011 Lecture 5 Thursday 6/10/2011 CLINICAL CORRELATION Disaccharidase Deficiency Intestinal disaccharidase deficiencies are encountered relatively frequently in humans. Deficiency can be present in one enzyme or several enzymes for a variety of reasons (genetic defect, physiological decline with age, or the result of "injuries" to the mucosa). Of the disaccharidases, lactase is the most common enzyme with an absolute or relative deficiency, which is experienced as milk intolerance. The consequences of an inability to hydrolyze lactose in the upper small intestine are inability to absorb lactose and bacterial fermentation of ingested lactose in the lower small intestine. Bacterial fermentation results in the production of gas (distension of gut and flatulence) and osmotically active solutes that draw water into the intestinal lumen (diarrhea). The lactose in yogurt has already been partially hydrolyzed during the fermentation process of making yogurt. Thus individuals with lactase deficiency can often tolerate yogurt better than unfermented dairy products. The enzyme lactase is commercially available to pretreat milk so that the lactose is hydrolyzed. 3) Absorption of Amino Acids and Peptides Dietary proteins are, with very few exceptions, not absorbed. Rather, they must be digested into amino acids or di- and tripeptides first, through the action of gastric and pancreatic proteases. The brush border of the small intestine is equipped with a family of peptidases. Like lactase and maltase, these peptidases are integral membrane proteins rather than soluble enzymes. They function to further the hydrolysis of lumenal peptides, converting them to free amino acids and very small peptides. These endproducts of digestion, formed on the surface of the enterocyte, are ready for absorption. a) Absorption of Amino Acids The mechanism by which amino acids are absorbed is conceptually identical to that of monosaccharides. The lumenal plasma membrane of the absorptive cell bears at least four sodium-dependent amino acid transporters - one each for acidic, basic, neutral and amino acids. These transporters bind amino acids only after binding sodium. The fully loaded transporter then undergoes a conformational change that dumps sodium and the amino acid into the cytoplasm, followed by its reorientation back to the original form. Thus, absorption of amino acids is also absolutely dependent on the electrochemical gradient of sodium across the epithelium. Further, absorption of amino acids, like that of monosaccharides, contributes to generating the osmotic gradient that drives water absorption. The basolateral membrane of the enterocyte contains additional transporters which export amino acids from the cell into blood. These are not dependent on sodium gradients. 48 Prof.Dr H.D.El-Yassin 2011 Lecture 5 Thursday 6/10/2011 CLINICAL CORRELATION Neutral Amino Aciduria (Hartnup Disease) Transport functions, like enzymatic functions, are subject to modification by mutations. An example of a genetic lesion in epithelial amino acid transport is Hartnup disease, named after the family in which the disease entity resulting from the defect was first recognized. The disease is characterized by the inability of renal and intestinal epithelial cells to absorb neutral amino acids from the lumen. In the kidney, in which plasma amino acids reach the lumen of the proximal tubule through the ultrafiltrate, the inability to reabsorb amino acids manifests itself as excretion of amino acids in the urine (amino aciduria). The intestinal defect results in malabsorption of free amino acids from the diet. Therefore the clinical symptoms of patients with this disease are mainly those due to essential amino acid and nicotinamide deficiencies. The pellagra-like features are explained by a deficiency of tryptophan, which serves as precursor for nicotinamide. Investigations of patients with Hartnup disease revealed the existence of intestinal transport systems for di- or tripeptides, which are different from the ones for free amino acids. The genetic lesion does not affect transport of peptides, which remains as a pathway for absorption of protein digestion products b) Absorption of Peptides There is virtually no absorption of peptides longer than four amino acids. However, there is abundant absorption of di- and tripeptides in the small intestine. These small peptides are absorbed into the small intestinal epithelial cell by cotransport with H+ ions via a transporter called PepT1. Once inside the enterocyte, the vast bulk of absorbed di- and tripeptides are digested into amino acids by cytoplasmic peptidases and exported from the cell into blood. Only a very small number of these small peptides enter blood intact. c) Absorption of Intact Proteins Absorption of intact proteins occurs only in a few circumstances. Normal" enterocytes do not have transporters to carry proteins across the plasma membrane and they certainly cannot permeate tight junctions. One important exception is that for a very few days after birth, neonates have the ability to absorb intact proteins. This ability, which is rapidly lost, is of immense importance because it allows the newborn animal to acquire passive immunity by absorbing immunoglobulins in colostral milk. The small intestine rapidly loses the capacity to absorb intact proteins. 4) Absorption of Lipids The bulk of dietary lipid is neutral fat or triglyceride, composed of a glycerol backbone with each carbon linked to a fatty acid. Additionally, most foodstuffs contain phospholipids, sterols like cholesterol and many minor lipids, including fat-soluble vitamins. In order for the triglyceride to be absorbed, two processes must occur: Large aggregates of dietary triglyceride, which are virtually insoluble in an aqueous environment, must be broken down physically and held in suspension - a process called emulsification. Triglyceride molecules must be enzymatically digested to yield monoglyceride and fatty acids, both of which can efficiently diffuse into the enterocyte The key players in these two transformations are bile salts and pancreatic lipase, both of which are mixed with chyme and act in the lumen of the small intestine. 49 Prof.Dr H.D.El-Yassin 2011 Lecture 5 Thursday 6/10/2011 Digestion and absorption of lipids Changes in physical state during triacylglycerol digestion. Abbreviations: TG, triacylglycerol; DG, diacylglycerol; MG, monoacylglycerol; FA, fatty acid CLINICAL CORRELATION A--Lipoproteinemia A-b-lipoproteinemia is an autosomal recessive disorder characterized by the absence of all lipoproteins containing apo--lipoprotein, that is, chylomicrons, very low density lipoproteins (VLDLs), and low density lipoproteins (LDLs). Serum cholesterol is extremely low. This defect is associated with severe malabsorption of triacylglycerol and lipid-soluble vitamins (especially tocopherol and vitamin E) and accumulation of apo B in enterocytes and hepatocytes. The defect does not appear to involve the gene for apo B, but rather one of several proteins involved in processing of apo B in liver and intestinal mucosa, or in assembly and secretion of triacylglycerol-rich lipoproteins, that is, chylomicrons and VLDLs from these tissues, respectively. 50 Prof.Dr H.D.El-Yassin 2011 Lecture 5 Thursday 6/10/2011 5) Absorption of Minerals and Metals The vast bulk of mineral absorption occurs in the small intestine. The best-studied mechanisms of absorption are clearly for calcium and iron, deficiencies of which are significant health problems throughout the world. a) Calcium The quantity of calcium absorbed in the intestine is controlled by how much calcium has been in the diet during recent periods of time. Calcium is absorbed by two distinct mechanisms: 1. Active, transcellular absorption occurs only in the duodenum when calcium intake has been low. This process involves import of calcium into the enterocyte, transport across the cell, and export into extracellular fluid and blood. The rate limiting step in transcellular calcium absorption is transport across the epithelial cell, which is greatly enhanced by the carrier protein calbindin, the synthesis of which is totally dependent on vitamin D. 2. Passive, paracellular absorption occurs in the jejunum and ileum, and, to a much lesser extent, in the colon when dietary calcium levels have been moderate or high. In this case, ionized calcium diffuses through tight junctions into the basolateral spaces around enterocytes, and hence into blood. Such transport depends on having higher concentrations of free calcium in the intestinal lumen than in blood. b) Phosphorus Phosphorus is predominantly absorbed as inorganic phosphate in the upper small intestine. Phosphate is transported into the epithelial cells by cotransport with sodium, and expression of this (or these) transporters is enhanced by vitamin D. c) Iron Iron homeostasis is regulated at the level of intestinal absorption, and it is important that adequate but not excessive quantities of iron be absorbed from the diet. Inadequate absorption can lead to iron-deficiency disorders such as anemia. On the other hand, excessive iron is toxic because mammals do not have a physiologic pathway for its elimination. 51 Prof.Dr H.D.El-Yassin 2011 Lecture 5 Thursday 6/10/2011 Iron is absorbed by villus enterocytes in the proximal duodenum. Efficient absorption requires an acidic environment. Ferric iron (Fe+++) in the duodenal lumen is reduced to its ferrous form through the action of a brush border ferrireductase. Iron is then co transported with a proton into the enterocyte via the divalent metal transporter DMT-1. This transporter is not specific for iron, and also transports many divalent metal ions. Once inside the enterocyte, iron follows one of two major pathways: Iron abundance states: iron within the enterocyte is trapped by incorporation into ferritin and hence, not transported into blood. When the enterocyte dies and is shed, this iron is lost. Iron limiting states: iron is exported out of the enterocyte via a transporter (ferroportin) located in the basolateral membrane. It then binds to the ironcarrier transferrin for transport throughout the body. d) Copper There appear to be two processes responsible for copper absorption: i) a rapid, low capacity system and ii) a slower, high capacity system, which may be similar to the two processes seen with calcium absorption. Many of the molecular details of copper absorption remain to be elucidated. Inactivating mutations in the gene encoding an intracellular copper ATPase have been shown responsible for the failure of intestinal copper absorption in Menkes disease. A number of dietary factors have been shown to influence copper absorption. For example, excessive dietary intake of either zinc or molybdenum can induce secondary copper deficiency states. e) Zinc Zinc homeostasis is largely regulated by its uptake and loss through the small intestine. Although a number of zinc transporters and binding proteins have been identified in villus epithelial cells, a detailed picture of the molecules involved in zinc absorption is not yet in hand. A number of nutritional factors have been identified that modulate zinc absorption. Certain animal proteins in the diet enhance zinc absorption. Phytates from dietary plant material (including cereal grains, corn, rice) chelate zinc and inhibit its absorption. Subsistence on phytate-rich diets is thought responsible for a considerable fraction of human zinc deficiencies. 52 Prof.Dr H.D.El-Yassin 2011 Lecture 6 Sunday 9/10/2011 Secretion and Absorption in the Large Intestine The large intestine is the last attraction in digestive tube and the location of the terminal phases of digestion. It functions in three processes: Recovery of water and electrolytes from ingesta: By the time ingesta reaches the terminal ileum, roughly 90% of its water has been absorbed, but considerable water and electrolytes like sodium and chloride remain and must be recovered by absorption in the large gut. Formation and storage of feces: Microbial fermentation: The large intestine of all species teems with microbial life. Those microbes produce enzymes capable of digesting many of molecules that to vertebrates are indigestible, cellulose being a premier example. The extent and benefit of fermentation also varies greatly among species. Absorption, Secretion and Formation of Feces in the Large Intestine 1. Absorption: water, sodium ions and chloride ions 2. Secretion: bicarbonate ions and mucus Water, as always, is absorbed in response to an osmotic gradient. The mechanism responsible for generating this osmotic pressure is essentially identical to what was seen in the small intestine - sodium ions are transported from the lumen across the epithelium by virtue of the epithelial cells having very active sodium pumps on their basolateral membranes and a means of absorbing sodium through their lumenal membranes. The colonic epithelium is actually more efficient at absorbing water than the small intestine and sodium absorption in the colon is enhanced by the hormone aldosterone. Chloride is absorbed by exchange with bicarbonate. The resulting secretion of bicarbonate ions into the lumen aids in neutralization of the acids generated by microbial fermentation in the large gut. 53 Prof.Dr H.D.El-Yassin 2011 Lecture 6 Sunday 9/10/2011 Model for electrogenic NaCl absorption in the large intestine This Na+ flux is electrogenic; that is, it is associated with an electrical current, and it can be inhibited by the diuretic drug amiloride at micromolar concentrations Microbial Fermentation Fermentation is the enzymatic decomposition and utililization of foodstuffs, particularly carbohydrates, by microbes The large intestine does not produce its own digestive enzymes, but contains huge numbers of bacteria which have the enzymes to digest and utilize many substrates. In all animals, two processes are attributed to the microbial flora of the large intestine: 1. Digestion of carbohydrates not digested in the small intestine 2. Synthesis of vitamin K and certain B vitamins Cellulose is common constituent in the diet of many animals, including man, but no mammalian cell is known to produce a cellulase. Several species of bacteria in the large bowel synthesize cellulases and digest cellulose. Importantly, the major end products of microbial digestion of cellulose and other carbohydrates are volatile fatty acids, lactic acid, methane, hydrogen and carbon dioxide. Fermentation is thus the major source of intestinal gas. Volatile fatty acids (acetic, proprionic and butyric acids) generated from fermentation can be absorbed by diffusion in the colon. Synthesis of vitamin K by colonic bacteria provides a valuable supplement to dietary sources and makes clinical vitamin K deficiency rare. Similarly, formation of B vitamins by the microbial flora in the large intestine is useful to many animals. They are not absorbed in the large intestine, but are present in feces. 54 Prof.Dr H.D.El-Yassin 2011 Lecture 6 Sunday 9/10/2011 Intestinal Gas Production A considerable amount of gas is present in the gastrointestinal contents of all animals. Five gases constitute greater than 99% of the gases passed: N2, O2, CO2, H2 and methane. None of these gases has an odor, and the characteristic odor of feces is due to very small quantities of a few other gases, including hydrogen sulfide. There are three principal sources of the five major intestinal gases: 1. Air swallowing is the major source of gas in the stomach. 2. Intraluminal generation of gases results from two major processes; First, in the proximal intestine, the interaction of hydrogen and bicarbonate ions (principally from gastric and pancreatic secretions) leads to generation of CO2. The amount of gas generated by this pathway is not great, because the lumenal contents do not contain carbonic anhydrase and the dissociation of H2CO3 is thus quite slow. Additionally, most of the CO2 produced in this way is absorbed into blood. The second and much more productive source of gas is fermentation by colonic bacteria. Microbes appear to be the sole source of all of the hydrogen and methane produced in the intestine. A variety of fruits and vegetables contain polysaccharides that are not digested in the small intestine and lead to voluminous gas production by microbes. Indeed, the primary medical treatment for excessive gas production is dietary manipulation to eliminate foodstuffs that the individual cannot digest and absorb. The Gastrointestinal Barrier The gastrointestinal mucosa forms a barrier between the body and a lumenal environment which not only contains nutrients, but is loaded with potentially hostile microorganisms and toxins. The challenge is to allow efficient transport of nutrients across the epithelium while rigorously excluding passage of harmful molecules and organisms into the animal. The exclusionary properties of the gastric and intestinal mucosa are referred to as the "gastrointestinal barrier". The gastrointestinal barrier is often discussed as having two components: 1. The intrinsic barrier is composed of the epithelial cells lining the digestive tube and the tight junctions that tie them together. 2. The extrinsic barrier consists of secretions and other influences that are not physically part of the epithelium, but which affect the epithelial cells and maintain their barrier function. a. Mucus and Bicarbonate 55 Prof.Dr H.D.El-Yassin 2011 Lecture 6 Sunday 9/10/2011 The entire gastrointestinal epithelium is coated with mucus, which serves an important role in mitigating shear stresses on the epithelium and contributes to barrier function in several ways. The abundant carbohydrates on mucin molecules bind to bacteria, which aids in preventing epithelial colonization and, by causing aggregation, accelerates clearance. Diffusion of hydrophilic molecules is considerably lower in mucus than in aqueous solution, which is thought to retard diffusion of a variety of damaging chemicals, including gastric acid, to the epithelial surface. b. Hormones and Cytokines Normal proliferation of gastric and intestinal epithelial cells, as well as proliferation in response to such injury as ulceration, is known to be affected by a large number of endocrine and paracrine factors. Several of the enteric hormones are known to enhance rates of proliferation. Different forms of injury to the epithelium can lead to either enhanced or suppressed rates of cell proliferation. Prostaglandins, particularly prostaglandin E2 and prostacyclin, have long been known to have "cytoprotective" effects on the gastrointestinal epithelium. A common clinical correlate in many mammals is that use of aspirin and other non-steroidal antiinflammatory drugs (NSAIDs) which inhibit prostaglandin synthesis is commonly associated with gastric erosions and ulcers. c. Antibiotic Peptides and Antibodies 56 Prof.Dr H.D.El-Yassin 2011 Lecture 6 Sunday 9/10/2011 Digestive Disorders 1. Stomach and Intestine Peptic ulcers Peptic ulcer is a general term that refers to ulcers occurring in the lower esophagus, the stomach, or the duodenum (upper part of the small intestine). What is the difference between a duodenal ulcer and a gastric ulcer? A duodenal ulcer is a break in the lining of the upper part of the small intestine (the duodenum); a gastric ulcer is a break in the lining of the stomach. What causes duodenal and gastric ulcers? Duodenal ulcers are much more common than gastric ulcers. The primary cause of duodenal ulcers is increased production of acid by the stomach. Gastric ulcers, on the other hand, are thought to be caused by changes in the stomach lining that make it more susceptible to damage by the acid normally produced by the stomach. Factors in the development of peptic ulcers include: Helicobacter pylori Research shows that most ulcers develop as a result of infection with bacterium called Helicobacter pylori (H. pylori). Smoking Studies show smoking increases the chances of getting an ulcer, slows the healing process of existing ulcers, and contributes to ulcer recurrence. 57 Prof.Dr H.D.El-Yassin 2011 Lecture 6 Sunday 9/10/2011 Caffeine Caffeine seems to stimulate acid secretion in the stomach, which can aggravate the pain of an existing ulcer. However, the stimulation of stomach acid cannot be attributed solely to caffeine. Alcohol Although no proven link has been found between alcohol consumption and peptic ulcers, ulcers are more common in people who have cirrhosis of the liver, a disease often linked to heavy alcohol consumption. Stress Mucos HCO3 content creates a "micro-environment" around surface cells to prevent acid damage, but its secretion is inhibited by adrenergic input (prominent in stress!) Acid and pepsin It is believed that the stomach's inability to defend itself against the powerful digestive fluids, hydrochloric acid and pepsin, contributes to ulcer formation. nonsteroidal anti-inflammatory drugs (NSAIDs) These drugs (such as aspirin, ibuprofen, and naproxen sodium) make the stomach vulnerable to the harmful effects of acid and pepsin. 2. Bile and the Biliary System a. Gallstones (Cholelithiasis) There are two major types of gallstones, which form due to distinctly different pathogenetic mechanisms. 1. Cholesterol Stones About 90% of gallstones are of this type. These stones can be almost pure cholesterol or mixtures of cholesterol and substances such as mucin. The key event leading to formation and progression of cholesterol stones is precipitation of cholesterol in bile. There are clearly important genetic determinants for cholesterol stone formation. There is also an important gender bias in development of stones - the prevalence in adult females is two to three times that seen in males and use of contraceptive steroids is a risk factor for development of gallstones. 2. Pigment Stones Roughly 10% of human gallstones are pigment stones composed of large quantities of bile pigments, along with lesser amounts of cholesterol and calcium salts. The most important risk factor for development of these stones is chronic hemolysis from almost any cause - bilirubin is a major constituent of these stones. Additionally, some forms of pigment stones are associated with bacterial infections. Apparently, some bacteria release deconjugate bilirubin, leading to precipitation as calcium salts. 58 Prof.Dr H.D.El-Yassin 2011 Lecture 6 Sunday 9/10/2011 b. Jaundice Jaundice, is yellowing of the skin, sclera (the white of the eyes) and mucous membranes caused by increased levels of bilirubin in the human body. Usually the concentration of bilirubin in the blood must exceed 23mg/dL for the coloration to be easily visible. Jaundice comes from the French word jaune, meaning yellow. Causes of jaundice When red blood cells die, the heme in their hemoglobin is converted to bilirubin in the spleen. The bilirubin is processed by the liver, enters bile and is eventually excreted through faeces. Consequently, there are three different classes of causes for jaundice. Pre-hepatic or hemolytic causes, where too many red blood cells are broken down, hepatic causes where the processing of bilirubin in the liver does not function correctly, and post-hepatic or extrahepatic causes, where the removal of bile is disturbed. 1. Pre-hepatic Pre-hepatic (or hemolytic) jaundice is caused by anything which causes an increased rate of hemolysis (breakdown of red blood cells). Malaria can cause jaundice. Certain genetic diseases, such as glucose 6phosphate dehydrogenase deficiency can lead to increase red cell lysis and therefore hemolytic jaundice. Defects in bilirubin metabolism also present as jaundice. 2. Hepatic Hepatic causes include acute hepatitis, hepatotoxicity and alcoholic liver disease. Jaundice commonly seen in the newborn baby is another example of hepatic jaundice. 59 Prof.Dr H.D.El-Yassin 2011 Lecture 6 Sunday 9/10/2011 Neonatal jaundice Neonatal jaundice is usually harmless: this condition is often seen in infants around the second day after birth, lasting till day 8 in normal births, or to around day 14 in premature births. Serum bilirubin normally drops to a low level without any intervention required: the jaundice is presumably a consequence of metabolic and physiological adjustments after birth. Infants with neonatal jaundice are typically treated by exposing them to high levels of colored light to break down the bilirubin. Lecture 6 Sunday 9/10/2011 This works due to a photo oxidation process occurring on the bilirubin in the subcutaneous tissues of the neonate. Light energy creates isomerization of the bilirubin and consequently transformation into compounds that the new born can excrete via urine and stools. 3. Post-hepatic Post-hepatic (or obstructive) jaundice, also called cholestasis, is caused by an interruption to the drainage of bile in the biliary system. The most common causes are gallstones in the common bile duct and pancreatic cancer in the head of the pancreas. The van den Bergh test: When a mixture of sulphanic acid, hydrochloric acid and sodium nitrite (diazo reagent) is added to serum containing an excess of biliriubin glucuronide a reddish-violet color results, the maximum color intensity being reached within 30 seconds (direct reaction) (for hepatic and post hepatic jaundice) When the same above reagents are mixed with serum containing an excess of billirubin itself or bilirubi-protien complex no color develops until alcohol is added, then the reddish-violet color appears.(indirect reaction) (for pre hepatic jaundice) Note: the addition of alcohol solvent provides the means of solution for the water insoluble bilirubin which is thus enabled to react with the diazo reagent c. Cirrhosis Cirrhosis is characterized anatomically by widespread nodules in the liver combined with fibrosis. The fibrosis and nodule formation causes distortion of the normal liver architecture which interferes with blood flow through the liver. Cirrhosis can also lead to an inability of the liver to perform its biochemical functions. Causes of Cirrhosis Alcoholic liver disease Chronic viral hepatitis B, C and D Chronic autoimmune hepatitis Inherited metabolic diseases Chronic bile duct diseases Chronic congestive heart failure infections Parasitic infections liver inflammation that can be caused by fatty liver long term exposure to toxins or drugs 60 Prof.Dr H.D.El-Yassin 2011 Lecture 6 Sunday 9/10/2011 3. Intestine Diarrhea is an increase in the volume of stool or frequency of defecation. It is one of the most common clinical signs of gastrointestinal disease, but also can reflect primary disorders outside of the digestive system There are numerous causes of diarrhea, but in almost all cases, this disorder is a manifestation of one of the four basic mechanisms described below. 1. Osmotic Diarrhea Absorption of water in the intestines is dependent on adequate absorption of solutes. If excessive amounts of solutes are retained in the intestinal lumen, water will not be absorbed and diarrhea will result. Osmotic diarrhea typically results from one of two situations: Ingestion of a poorly absorbed substrate: The offending molecule is usually a carbohydrate or divalent ion. Common examples include mannitol or sorbitol, epson salt (MgSO4) and some antacids (MgOH2). Malabsorption: Inability to absorb certain carbohydrates is the most common deficit in this category of diarrhea, but it can result virtually any type of malabsorption. A common example is lactose intolerance resulting from a deficiency in the brush border enzyme lactase. In such cases, a moderate quantity of lactose is consumed (usually as milk), but the intestinal epithelium is deficient in lactase, and lactose cannot be effectively hydrolyzed into glucose and galactose for absorption. The osmotically-active lactose is retained in the intestinal lumen, where it "holds" water. A distinguishing feature of osmotic diarrhea is that it stops after the patient is fasted or stops consuming the poorly absorbed solute. 2. Secretory Diarrhea Large volumes of water are normally secreted into the small intestinal lumen, but a large majority of this water is efficiently absorbed before reaching the large intestine. Diarrhea occurs when secretion of water into the intestinal lumen exceeds absorption. Many millions of people have died of the secretory diarrhea associated with cholera. The responsible organism, Vibrio cholerae, produces cholera toxin, which strongly activates adenylyl cyclase, causing a prolonged increase in intracellular concentration of cyclic AMP within crypt enterocytes. This change results in prolonged opening of the chloride channels that are instrumental in secretion of water from the crypts, allowing uncontrolled secretion of water. Exposure to toxins from several other types of bacteria (e.g. E. coli heatlabile toxin) induce the same series of steps and massive secretory 61 Prof.Dr H.D.El-Yassin 2011 Lecture 6 Sunday 9/10/2011 diarrhea that is often lethal unless the person or animal is aggressively treated to maintain hydration. In addition to bacterial toxins, a large number of other agents can induce secretory diarrhea by turning on the intestinal secretory machinery, including: some laxatives hormones secreted by certain types of tumors (e.g. vasoactive intestinal peptide) a broad range of drugs (e.g. some types of asthma medications, antidepressants, cardiac drugs) certain metals, organic toxins, and plant products (e.g. arsenic, insecticides, mushroom toxins, caffeine) In most cases, secretory diarrheas will not resolve during a 2-3 day fast. 3. Inflammatory and Infectious Diarrhea The epithelium of the digestive tube is protected from insult by a number of mechanisms constituting the gastrointestinal barrier, but like many barriers, it can be breached and often associated with widespread destruction of absorptive epithelium. In such cases, absorption of water occurs very inefficiently and diarrhea results. Examples of pathogens frequently associated with infectious diarrhea include: Bacteria: Salmonella, E. coli, Campylobacter Viruses: rotaviruses Protozoa: 4. Diarrhea Associated with Deranged Motility In order for nutrients and water to be efficiently absorbed, the intestinal contents must be adequately exposed to the mucosal epithelium and retained long enough to allow absorption. Disorders in motility than accelerate transit time could decrease absorption, resulting in diarrhea even if the absorptive process per se was proceeding properly. 62 Prof.Dr H.D.El-Yassin 2011