Exam 2 - HCC Southwest College
advertisement

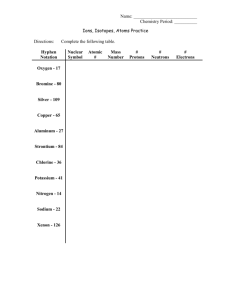
Houston Community College System Chemistry 1405 SAMPLE EXAM # 2 1 HOUSTON COMMUNITY COLLEGE CHEM 1405 Name: _____________________ Exam # 2 (Chap. 5- 8) Score: PART I – Multiple choice: (3 points each) 1) What is the subatomic particle having a negligible mass and a negative charge? A) electron B) neutron C) proton D) quark 2) What is the general term that refers to either visible or invisible radiant energy? A) continuous spectrum B) emission spectrum C) line spectrum D) light 3) How many neutrons are in the nucleus of an atom of 118 50 Sn ? A) 50 B) 68 C) 118 D) 168 4) Given that the only naturally occurring isotope of sodium is 23Na, what is its isotopic mass? A) 11.00 amu B) 11.99 amu C) 12.00 amu D) 22.99 amu 5) What is the term for the minimum amount of energy to remove an electron from a neutral atom in the gaseous state? A) atomic energy B) electron energy C) ionization energy D) nuclear energy C) Br D) all of the above 6) Which of the following is a representative element? A) B B) Be 7) Which fourth period transition element has the highest atomic number? A) Ca B) Cd C) Kr D) Zn 8) Which of the following elements are fourth period semimetals? A) Si and Ge B) Ge and As C) Sb and Te D) Po and At C) Ca D) none of the above 9) Which of the following is an alkali metal? A) Al B) Fe 10) Predict the density for rhodium, Rh, given the density of cobalt, Co, (8.89 g/cm3) and iridium, Ir, (22.65 g/cm3). A) 2.01 g/cm3 B) 6.88 g/cm3 C) 13.76 g/cm3 D) 15.77 g/cm3 11) The compound Au2Se3 is classified as which of the following? A) binary ionic B) ternary ionic C) binary molecular 2 D) binary acid 12) What is the Stock system name for Cu+? A) copper ion B) copper(I) ion C) cupric ion D) cuprous ion C) nitride ion D) nitrite ion 13) What is the systematic name for N3-? A) ammonium ion B) nitrogen ion 14) What is the chemical formula for the manganese(II) ion? A) Mg2+ B) Mn2+ C) Mg3+ D) Mn3+ 15) What is the predicted ionic charge of an element in Group IIA/2? A) 2+ B) 6+ C) 2- D) 6- C) chromate ion D) chromium ion 16) What is the systematic name for CrO42-? A) chromide ion B) chromite ion 17) What is the name of a reaction in which two cations in different compounds exchange anions? A) combination B) decomposition C) double replacement D) neutralization 18) Which of the following is evidence for a chemical reaction? A) A gas is detected. C) A flame is observed. B) A precipitate is formed. D) all of the above 19) Which of the statements below best describes the following reaction? Na2CO3(s) Na2O(s) + CO2(g) A) Solid sodium carbonate is heated to give solid sodium oxide and carbon dioxide gas. B) Sodium carbonate decomposes to sodium oxide and carbon dioxide. C) Sodium carbonate decomposes to sodium oxide and carbon dioxide gas. D) Sodium carbonate is heated to give sodium oxide and carbon dioxide. 20) What is the coefficient of oxygen gas after balancing the following equation? A) 1 ___P(s) + ___O2(g) → ___P2O3(s) B) 2 C) 3 3 D) 5 PART II – Show All Your Work: (8 points each) 1) What is the predicted product from the following reaction? Balance this equation. What kind of reaction is it? Ca(s) + O2(g) 2) Which of the following metals does not react with aqueous Ni(NO3)2? Why? Partial Activity Series: Mg > Al > Ni > (H) > Cu 3) What is the name of each compound? a. Ca(OH)2 b. KCl c. AlBr3 d. NaF 4) What are the chemical formulas for oxide ion and iodide ion? 5) What is the core notation for the electron configuration of an aluminum atom? BONUS QUESTION – Show All Your Work: (10 points) What is the chemical formula of each of the following compounds? a. Sulfate ion b. Barium Chromate c. Aluminum Chloride d. Magnesium Oxide 4 5 Answer Key Part I- Multiple Choice 1) A 8) B 15) A 2) D 9) D 16) C 3) B 10) D 17) C 4) D 11) A 18) D 5) C 12) B 19) A Part II- Show Your Work 1) CaO, Combination Reaction 2) Cu, because it is not as active as Ni 3) a. Calcium Hydroxide b. Potassium Chloride c. Aluminum Bromide d. Sodium Fluoride 4) O2-, I5) [Ne] 3s2 3p1 Bonus Question: a. b. c. d. SO42 BaCrO4 AlCl3 MgO 6 6) D 13) C 20) C 7) D 14) B