Solving Thermodynamics Problems
advertisement
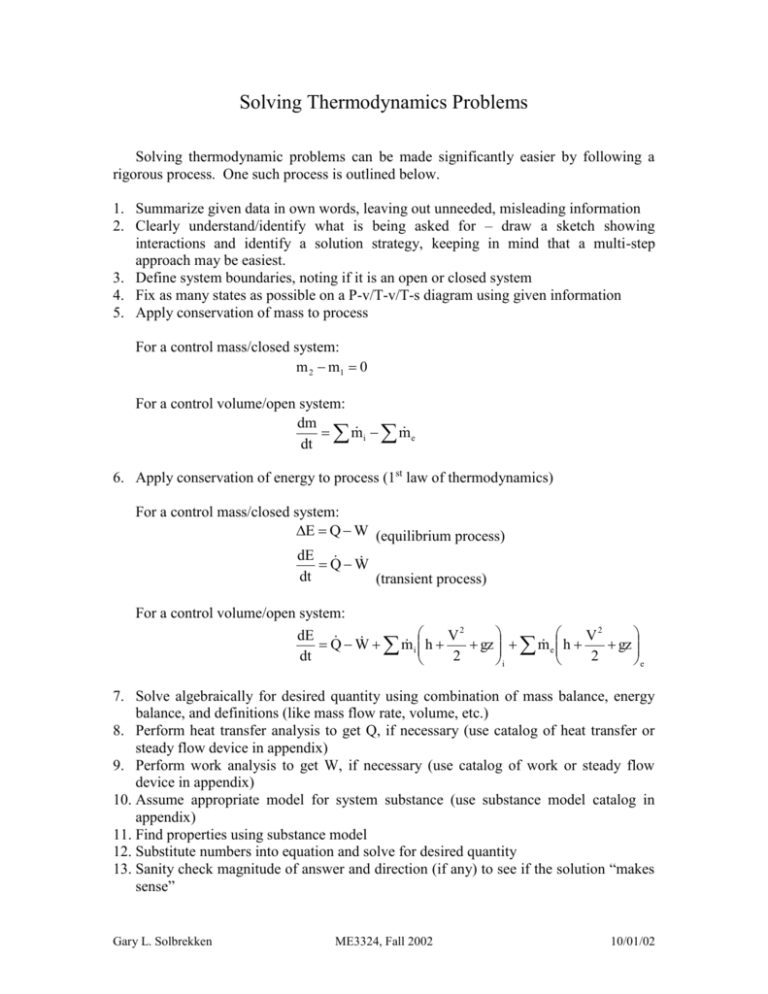
Solving Thermodynamics Problems Solving thermodynamic problems can be made significantly easier by following a rigorous process. One such process is outlined below. 1. Summarize given data in own words, leaving out unneeded, misleading information 2. Clearly understand/identify what is being asked for – draw a sketch showing interactions and identify a solution strategy, keeping in mind that a multi-step approach may be easiest. 3. Define system boundaries, noting if it is an open or closed system 4. Fix as many states as possible on a P-v/T-v/T-s diagram using given information 5. Apply conservation of mass to process For a control mass/closed system: m 2 m1 0 For a control volume/open system: dm i m e m dt 6. Apply conservation of energy to process (1st law of thermodynamics) For a control mass/closed system: E Q W (equilibrium process) dE QW dt (transient process) For a control volume/open system: dE V2 V2 i h e h Q W m gz m gz dt 2 2 i e 7. Solve algebraically for desired quantity using combination of mass balance, energy balance, and definitions (like mass flow rate, volume, etc.) 8. Perform heat transfer analysis to get Q, if necessary (use catalog of heat transfer or steady flow device in appendix) 9. Perform work analysis to get W, if necessary (use catalog of work or steady flow device in appendix) 10. Assume appropriate model for system substance (use substance model catalog in appendix) 11. Find properties using substance model 12. Substitute numbers into equation and solve for desired quantity 13. Sanity check magnitude of answer and direction (if any) to see if the solution “makes sense” Gary L. Solbrekken ME3324, Fall 2002 10/01/02 Appendix This appendix contains a series of catalogs for common parameters that are needed in solving thermodynamics problems. The lists are not intended to be exhaustive, nor is the information contained in the catalogs complete. For a complete discussion of a particular entry, a reference from the Cengel and Turner (C&T) book is included with the chapter and section identified. Note that the symbols used take on the context of the problem. Again, the user should consult C&T for details. Catalog of Heat Transfer Heat Transfer Mode Conduction (C & T ch 15-16) Equation kA dT Q dx Q hA Twall Tambient F A T 4 T 4 Q Convection (C & T ch 17 – 18) Radiation (C & T ch 19) 12 1 2 Catalog of Work Work Mode Electric (C & T ch 4.2) Spring (C & T ch 4.3.3) Equation W VIt 1 W k x 22 x12 2 W 2n Shaft (C & T ch 4.3.2) Expansion/Compression (C & T ch 4.3.1) W PdV Bar Deformation (C & T ch 4.3.4) W A o L o d Surface Tension (C&T ch 4.3.4) W dA Catalog of Steady Flow Devices Device Conversion Process Nozzle (C&T ch 5.4.1) Flow energy(T, P) to KE Diffuser KE to flow energy (T, P) Turbine (C&T ch 5.4.2) Flow energy (T, P) to work Compressor Work to flow energy (T, P) Pump Work to flow energy (P) Throttle Device (C&T ch Relieve pressure 5.4.3) Heat Exchanger (C&T ch Transfer heat between flow 5.4.4) streams Gary L. Solbrekken ME3324, Fall 2002 Typical Assumptions S.S., adiabatic, no work, PE = 0 S.S, adiabatic, KE PE 0 S.S., adiabatic, no work, KE PE 0 S.S., no work, KE PE 0, external surfaces adiabatic 10/01/02 Catalog of Substance Models Substance Model Application Domain Characteristics Property Tables – solid, Whenever experimental Real data, so this is ideal as liquid, vapor (C&T ch 3.6) data is available for long as the experimental substance of interest conditions used to make table are broadly applicable to the application. Incompressible – liquid Most liquids and processes Properties are approximated (C&T ch 3.6.3 and ch 3.11) where volume expansion is by the saturated liquid not of interest (an example properties at the system of a mis-application would temperature. Specific heats be a natural convection are temperature dependent process) only. Cp = Cv = C du = CdT = f(T) y yf(T) h hf(T) + vf(T)*[p – psat(T)] Incompressible – solid Most solids and processes (C&T ch 3.6.3 and ch 3.11) where volume expansion is not of interest (an example of a mis-application would be a deformation/bar expansion process) Compressibility Chart Vapors that cannot be (Principle of Corresponding classified as an ideal gas States)- vapor (C & T ch 3.7) Ideal Gas (C & T ch 3.7, ch Special case vapor where PR 3.10) (P/Pcrit) is nearly 0. Gary L. Solbrekken ME3324, Fall 2002 Specific heats are temperature dependent only. Cp = Cv = C du = CdT = f(T) Assumes that the vapors of all substances are qualitatively similar, relative to their critical state. Scaling relative to the critical state allows the generalized compressibility chart to be used to find relation between P-v-T. Z = Pv/RT PR = P/Pcrit TR = T/Tcrit Allows use of ideal gas equation of state, PV = mRT. Also, specific heats are only temperature dependent, meaning energies are also only functions of temperature du = CvdT = f(T) dh = CpdT = f(T) 10/01/02