second midterm examination
advertisement
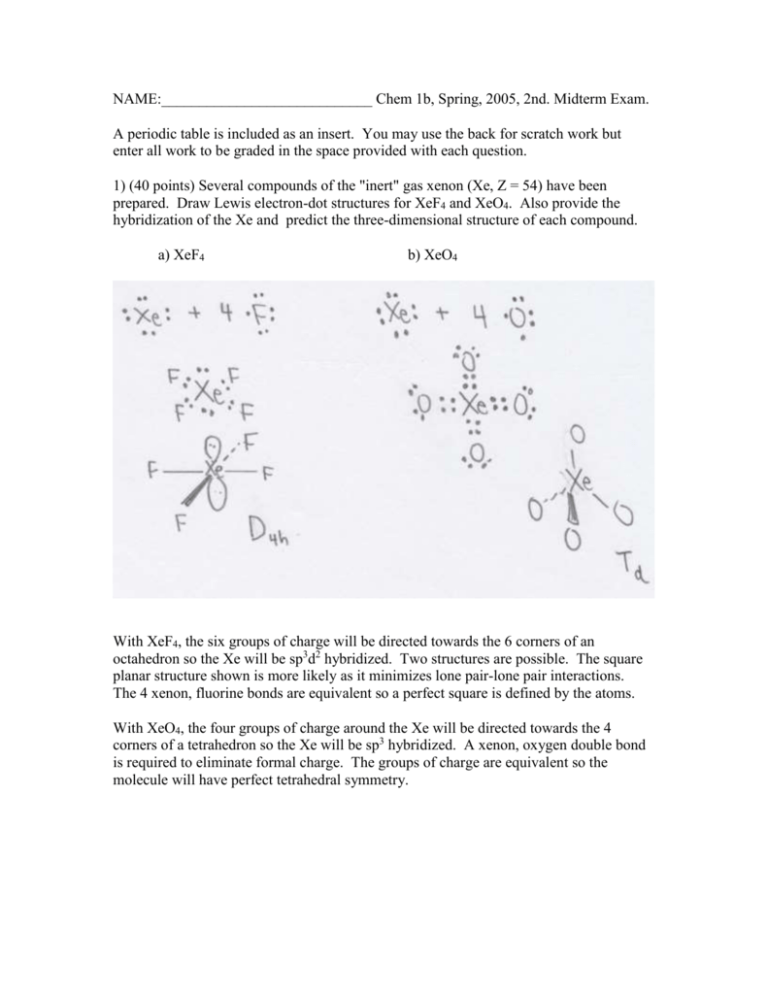
NAME:____________________________ Chem 1b, Spring, 2005, 2nd. Midterm Exam. A periodic table is included as an insert. You may use the back for scratch work but enter all work to be graded in the space provided with each question. 1) (40 points) Several compounds of the "inert" gas xenon (Xe, Z = 54) have been prepared. Draw Lewis electron-dot structures for XeF4 and XeO4. Also provide the hybridization of the Xe and predict the three-dimensional structure of each compound. a) XeF4 b) XeO4 With XeF4, the six groups of charge will be directed towards the 6 corners of an octahedron so the Xe will be sp3d2 hybridized. Two structures are possible. The square planar structure shown is more likely as it minimizes lone pair-lone pair interactions. The 4 xenon, fluorine bonds are equivalent so a perfect square is defined by the atoms. With XeO4, the four groups of charge around the Xe will be directed towards the 4 corners of a tetrahedron so the Xe will be sp3 hybridized. A xenon, oxygen double bond is required to eliminate formal charge. The groups of charge are equivalent so the molecule will have perfect tetrahedral symmetry. 2) (25 points) The structure of pyrrole, a very stable substance with the empirical formula C4H5N, is given below. a) Pyrrole has been shown to be completely planar. Use bonding theory to provide an explanation for its planarity. H N H H H H pyrrole Two factors account for the global planarity. First, all heavy atoms are sp2 hybridized. This leads to local planarity in the vicinity of each heavy atom. The Lewis structure implied by the covalent structure suggests sp3 hybridization for the nitrogen. However, if the N also has sp2 hybridization, then overlap of the 2pz unhybridized orbitals on all heavy atoms leads to a compensating stabilization. The delocalization of the pi electrons resulting from this overlap is the second factor required for global planarity. b) Draw a Lewis electron-dot structure of a stable isomer of pyrrole. Many isomers are possible. The structures of a few are given below. 3) (45 points) The nitrite anion, ONO-, is a well known species a) Draw a Lewis electron-dot structure(s) for nitrite. Consider resonance. Two resonance structures are possible. In both, a formal charge of -1 is on one of the oxygen atoms. The species is an anion and this formal charge is acceptable. The two structures are symmetrically equivalent and would make equal contributions to the valence bond (VB) wave function. b) A structural isomer of nitrite with the structural formula NOO- is possible. Draw a Lewis electron-dot structure for NOO-. Two resonance structures are possible. In both structures, all atoms have formal charge. However, in the right structure the nitrogen has a very unfavorable -1 formal charge. The right structure would make a smaller contribution to the VB wave function. c) Which species has the shorter nitrogen-oxygen bond length? Discuss. From the two equivalent resonance forms for nitrite, one concludes that the oxygen, nitrogen bond will be halfway between a double and a single bond. In the case of NOO-, the optimal structure, the one with a smaller formal charge on the nitrogen, has a nitrogen, oxygen double bond. If this structure dominates, the higher nitrogen, oxygen bond order would point to a shorter N, O bond length. However, the contribution of the other resonance structure with a N, O single bond would argue for a longer bond length. Hence, a qualitative discussion in this case does not lead to a definitive answer. A calculation is necessary. A HartreeFock calculation shows that the N, O bond lengths in the two species are nearly equal. d) Which species is more stable? Discuss. Nitrite, ONO-, is more stable. None of its resonance structures place formal charge on the nitrogen. The two equivalent resonance structures show a mechanism for extensive delocalization of the pi electrons. e) NOO- is a hypothetical species that has not been reported in the chemical literature. How could a Pomona student readily determine if it is a real species? An instrumental approach is beyond the skill of students. Pomona does not have a microwave spectrometer. Furthermore, the study of ions is very difficult in the gas phase. The complete absence of papers on NOO- shows that it would be present at very low concentrations if it exists at all. A direct, very accessible approach is the application of quantum mechanics. A Hartree-Fock calculation with a 3-21G* basis set with Spartan quickly shows that NOO- is a stable but high energy species. 4) (25 points) This problem addresses the thermodynamics of the gas-phase formation of ionic beryllium chloride. The first and second ionization energies of beryllium (Be, Z = 4) are 899 and 1757 kJ/mole. The electron affinity of chlorine (Cl, Z = 17) is 349 kJ/mole. E at 0 K for the step Be+2(g) + 2 Cl-(g) ClBeCl(g) is -2293 kJ. a) Calculate E at 0 K for the formation reaction Be(g) + 2 Cl(g) ClBeCl(g) Breakdown the overall reaction, Be(g) + 2 Cl(g) ClBeCl(g), into its components parts. You are using the path-independent feature of energy changes guaranteed by the First Law of Thermodynamics. E value(kJ/mole) BeBe 1IE(Be) +899 Be+1Be+2 1IE(Be+) = 2IE(Be) +1757 2 x (e- + ClCl-) 2 x (-EA(Cl)) -698 +2 2 Cl + Be ClBeCl E(Coulombic) -2293 (provided above) TOTAL -335 kJ/mole +1 b) The comparable number for the formation of gaseous MgCl2 is -485 kJ/mole. Suggest the most important factor for the difference in the values of E. The sizes of the ions (Be+2 is smaller) yields a larger electrostatic term. The greater stability for MgCl2 indicates that the electrostatic term reflecting the Coulombic attraction of the metal cation for the chloride anions is not dominant. The major factor here is the difference in the two ionization energies. Beryllium and magnesium belong to the same family so the trend with n applies. magnesium has much lower ionization energies and hence is more stable. 5) (15 points) Lise Nygaard determined the three-dimensional structure of pyrrole (the molecule examined in problem two) using microwave spectroscopy. Discuss why microwave spectroscopy is well suited to this structural determination. Several factors are important. First, the molecule has a permanent dipole moment. This is a necessary condition for microwave spectroscopy. Microwave spectroscopy is conducted in the gas phase at low pressures so that there are no constraints on the rotation of the molecule. Hence pyrrole, a covalently bonded molecule, must have be volatile. Microwave spectroscopy is associated with the overall rotation of the molecule and yields moments of inertia for the molecule from which the structural parameters can be extracted.