leopard-formatted
advertisement

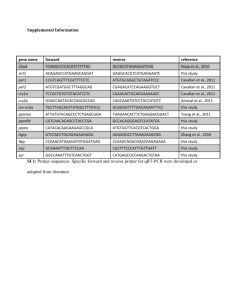
Behavioral and neurochemical changes in the zebrafish leopard strain Caio Maximino1,2*, Bruna Puty1, Karen Renata Matos Oliveira1, Anderson Manoel Herculano1,2** 1 Laboratório de Neuroendocrinologia, Instituto de Ciências Biológicas, Universidade Federal do Pará 2 Zebrafish Neuroscience Research Consortium * and ** - Corresponding authors Caio Maximino caio@ufpa.br Anderson Manoel Herculano herculano@ufpa.br Laboratório de Neuroendocrinologia Instituto de Ciências Biológicas Universidade Federal do Pará Av. Augusto Corrêa, 01 – 66075-110 1 – Belém, PA, Brazil Behavioral and neurochemical changes in the zebrafish leopard phenotype Abstract The zebrafish leopard phenotype (leo) displays abnormal pigmentation and shows increased anxietylike behavior. The neurochemical changes associated with this anxious phenotype are not known. Here, we demonstrate that leo show increased anxiety-like behavior in the light/dark box and in the novel tank test. This anxious phenotype is rescued by acute treatment with a dose of a serotonin reuptake inhibitor, fluoxetine, that is inactive in wild-type animals. Moreover, leo show decreased tissue levels of serotonin, increased serotonin turnover, and slightly increased monoamine oxidase activity. These results suggest that the anxious phenotype observed in leo zebrafish is caused by a decrease in serotonin uptake. The present work could open an important avenue in defining the neurochemical underpinning of natural variation in anxiety disorders. Keywords: strain, zebrafish, anxiety, scototaxis, serotonin uptake 2 Introduction Anxiety-like behavior in animals is controlled by a multiplicity of genes, leading to a plethora of strain effects in different organisms. In zebrafish, for example, increased anxiety-like behavior has been described in the leopard skin mutant, leo (Cachat et al. 2011, Egan et al. 2009), which present spots instead of stripes (Gilbert 2003). This phenotype has been described as caused by mutations in the cx41.8 gene which codes for a orthologue of the mammalian connexin 40 (Watanabe et al. 2006). Connexins are multimeric proteins which mediate cell-to-cell signaling by forming hemichannels and gap junctions; in the central nervous system, they mediate electrical coupling between neurons, metabolic coupling between glial cells, as well as the release of gliotransmitters such as glutamate and ATP (Sáez et al. 2003a, 2003b). Mice with astrocyte-targeted innactivation of connexin 43 show a phenotype of motor impairment and increased exploratory behavior in an open-field, but not in the elevated plus-maze (Frisch et al. 2003). Deletion of cx30, a protein which is expressed in astrocytes, increases centrophobism/thigmotaxis in mice associated with decreased serotonin turnover in the hippocampus (Dere et al. 2003). It is not known if the leopard phenotype results only from the cx41.8 mutation. Nonetheless, the increased anxiety-like behavior of this strain suggests that they may be an important tool in understanding the genetic underpinnings of anxiety and stress disorders. In spite of these initial observations, the neurochemical basis of this behavioral phenotype was not investigated. An important system in the control of anxiety is the serotonergic system (Maximino, 2012), suggesting an avenue of inquiry to interpret the neurochemical correlates of strain-dependent anxiety-like behavior in zebrafish. Here, we test whether this phenotype is generalized to other behavioral contexts thought to reflect anxiety/fear, and whether it is associated with changes in the serotonergic system. Methods 3 Drugs and reagents Fluoxetine was bought from Roche (São Paulo, Brazil). HPLC standards (5-HT, 5-HIAA and DHBA) and kynuramine hydrobromide were bought from Sigma-Aldrich (St. Louis, USA). HPLC-grade methanol was bought from Tedia (Fairfield, USA), and biotechnology-grade sodium dodecyl sulfate was bought from Amresco (Solon, USA). All other reagents were analytical-grade and bought from Synth (Diadema, Brazil). Animals Wild-type longfin (n = 20) and leopard mutant (n = 20) zebrafish, bought from the same local ornamental aquarium shop, were used in the experiments. Phenotypically, leo zebrafish were identified as homozygous t1 variants (Haffter et al., 1996; Watanabe et al., 2006), although this allele has been identified in Tü, and not WT, zebrafish. Animals were collectively maintained in 40 L tanks, separated by stripe pattern phenotype, for at least two weeks before onset of experiments. Tanks were kept at constant temperature (28 °C), oxygenation, and light cycle (14:10 LD photoperiod). Animals were fed daily with Alcon flake food, and twice a week with live brine shrimp. Animals were housed, handled and disposed of humanely in accordance to the American Fisheries Society's Guidelines for the Use of Fishes in Research (http://fisheries.org/docs/policy_useoffishes.pdf). Behavioral assays Each animal was subjected to both behavioral tests, in a randomized and balanced order. The maximum delay between tests was 3 min. Before testing, animals were anesthetized in ice-cold water, transferred to a wet surgical through and injected intraperitoneally with fluoxetine (5 mg/kg) or Cortland's salt solution (Perry et al. 1984). Light-dark preference (Scototaxis). Determination of drug effects on scototaxis were carried as 4 described elsewhere (Araújo et al. 2012). Briefly, after drug injection and effect onset animals were transferred to a black and white central compartment of a black and white tank (15 cm X 10 cm X 45 cm h X d X l) for a 3-min. acclimation period, after which the doors which delimit this compartment were removed and the animal was allowed to freely explore the apparatus for 15 min. The following variables were recorded: time on the white compartment: the time spent in the white half of the tank (percentage of the trial); white avoidance habituation: the cumulative habituation ratio (CHR; Stewart et al., 2013), that is, the modulus of the difference between the total time spent in the white compartment in the last three and the first three minutes of the assay. squares crossed: the number of 10 cm² squares crossed by the animal in the white compartment; locomotion habituation: the CHR between the total number of squares crossed in the last three and the first three minutes of the assay. latency to white: the amount of time the animal spends in the black compartment before its first entry in the white compartment (s); entries in white compartment: the number of entries the animal makes in the white compartment in the whole session; erratic swimming: the number of “erratic swimming” events, defined as a zig-zag, fast, unpredictable course of swimming of short duration; and freezing: the proportional duration of freezing events (in % of time in the white compartment), defined as complete cessation of movements with the exception of eye and operculae movements. thigmotaxis: the proportional duration of thigmotaxis events (in % of time in the white compartment), defined as swimming in a distance of 2 cm or less from the white compartment’s 5 walls. risk assessment: the number of “risk assessment” events, defined as a fast (<1 s) entry in the white compartment followed by re-entry in the black compartment, or as a partial entry in the white compartment (i.e., the pectoral fin does not cross the midline). Novel tank test (Geotaxis). The protocol for the novel tank diving test used was modified from Egan et al. (Egan et al. 2009). Briefly, after drug injection animals (n = 9-10 per treatment) were transferred to the test apparatus, which consisted of a 15 x 25 x 20 cm (width x length x height) tank lighted from above by two 25 W fluorescent lamps, producing an average of 120 lumens above the tank. As soon as the animals were transferred to the apparatus, a webcam was activated and behavioral recording begun. The webcam filmed the apparatus from the front, thus recording the animal’s lateral and vertical distribution. Animals were allowed to freely explore the novel tank for 6 minutes, after which they were removed from it and exposed to the scototaxis tank. Video files were later analyzed by experimenters blind to the treatment using X-Plo-Rat 2005 (http://scotty.ffclrp.usp.br), and the images were divided in a 3 x 3 grid composed of 10 cm² squares. The following variables were recorded: time on top: the time spent in the top third of the tank; bottom-dwelling habituation: the ratio of time spent in the top third of the tank in the sixth and in the first minute (Wong et al. 2010). squares crossed: the number of 10 cm² squares crossed by the animal during the entire session; erratic swimming: the number of “erratic swimming” events, defined as sharp changes in direction or velocity and repeated fast acceleration bouts (Kalueff et al., In press); freezing: the total duration of freezing events, defined as complete cessation of movements with the exception of eye and operculae movements. Nervous tissue parcellation 6 Brains of WT and leo animals were dissected on ice-cold magnesium- and calcium-free phosphatebuffered saline (MCF) after sacrifice and homogenized in 200 µl of cold solution containing HClO4 70% [0.2 N], 10 mg EDTA, 9.5 mg sodium metabissulfite. The homogenates were briefly centrifuged at 500 g for 5 min at 4 ºC, and supernatant aliquots were filtered through a 0.2 µm syringe filter. The resulting samples were frozen at -20 ºC for subsequent analysis in the HPLC. For the formation of synaptossomal (isolated synaptic terminals and associated mitochondria) pellets for monoamine oxidase assays, brains were transferred to 1 mL TBS 50 mM, sonicated, and centrifuged at 1,000 g for 10 min at 4 °C. The supernatant was then withdrawn and centrifuged at 17,000 g for 20 min at 4°C to form the synaptossomal pellet. HPLC analysis of monoamines Serotonin, 5-HIAA and 3,4-dihydroxybenzylamine (DHBA) (50 mg) were dissolved in 100 mL of eluting solution (HClO4 70% [0.2 N], 10 mg EDTA, 9.5 mg sodium metabissulfite) and frozen at -20 °C, to later be used as standards. The HPLC system consisted of a delivery pump (LC20-AT, Shimadzu), a 20 μL sample injector (Rheodyne), a degasser (DGA-20A5), and an analytical column (Shimadzu Shim-Pack VP-ODS, 250 x 4.6 mm internal diameter). The integrating recorder was a Shimadzu CBM-20A (Shimadzu, Kyoto, Japan). An electrochemical detector (Model L-ECD-6A) with glassy carbon was used at a voltage setting of +0.72 V, with a sensitivity set at 2 nA full deflection. The mobile phase consisted of a solution of 70 mM phosphate buffer (pH 2.9), 0.2 mM EDTA, 34.6765 mM SDS, 10% HPLC-grade methanol and 20% sodium metabisulfite to prevent oxidation of 5-HT. The column temperature was set at 17 °C, and the isocratic flow rate was 1.6 ml/min. 0.5 mL of extracellular fluid solution (ECF) was mixed with 0.5 mL of eluting solution, filtered through a 0.22 µm syringe filter, spiked with 0.22 µl of 2.27 mM DHBA (internal standard) and then injected into the HPLC system. Protein content was assessed in the same samples as described elsewhere (Zor & 7 Selinger, 1996) Monoamine oxidase activity The determination of MAO activity was done according to Weissbach et al. (1960). Synaptosomal preparations were suspended in a reaction buffer containing 110 µM of kynuramine hydrobromide (~2 x Km; Aldeco et al., 2011) in 0.5 M phosphate buffered at pH 7.4. After the addition of kynuramine, an initial reading was made at 360 nm using a Spectrum Meter SP2000 UV-VIS spectrophotometer, at a temperature of 25 ºC. Posterior readings were made at 5-min. intervals until the absorbance disappeared. Protein content was assessed in the same samples as described elsewhere (Zor & Selinger, 1996). Specific activity was defined as the amount of kynuramine consumed in one minute per mg of protein, using an extinction coefficient of 10,800 M-1 cm-1. Statistical analysis Tissue monoamine content and MAO activity data were analyzed by t-tests. Behavioral tests were analyzed using one-way parametric or non-parametric ANOVAs, followed by Tukey's or Dunn's posthoc tests whenever p < 0.05. Latency data for the light/dark test were transformed in survival curves, and the difference in median survival was analyzed by the Mantel-Cox test. Results leopard zebrafish displayed more bottom-dwelling in the novel tank test (Figure 1A; F[3, 36] = 8.028, p = 0.0003; Tukey's HSD: q = 4.97, p < 0.05). Fluoxetine (5 mg/kg) decreased bottom-dwelling in leo mutants, but not in WT zebrafish (Tukey's HSD: q = 5.052 [0 vs. 5 mg/kg, leo], q = 0.02857 [0 vs. 5 mg/kg, WT], q = 5.023 [5 mg/kg, leo vs. WT]). Nonetheless, albeit no differences were found between WT and leo in habituation (Tukey's HSD: q = 3.193), fluoxetine promoted habituation in WT, but not 8 leo zebrafish (Figure 1B; F[3, 39] = 11.87, p < 0.0001). No further differences between strains or drug effects were observed in this test (Figures 1C-1F). In the scototaxis test, leo mutants spent less time in the white compartment than their WT counterparts (F[3, 39] = 7.647, p = 0.0004; q = 5.152, Tukey's HSD; Figure 2A). Treatment with fluoxetine (5 mg/kg) reverted this phenotype in leo animals (q = 6.314, 0 vs. 5 mg/kg, leo), without effects on white avoidance in WT animals (q = 0.6424, Tukey's HSD). No differences between groups were observed in the latency to explore the white compartment ( ² [df = 3] = 4.154, p = 0.2453; Figure 2B) nor in the habituation of white compartment exploration (F[3, 39] = 1.218, p = 0.3173; Figure 2C). The number of entries in the white compartment (Figure 2D) and its habituation (Figure 2F), as well as the number of squares crossed in the white compartment (Figure 2E), were likewise not affected (F[3, 39] = 1.09; H < 3.9, NS). Although thigmotaxis (Figure 2G) did not differ between phenotypes (q = 2.648, Tukey's HSD), fluoxetine did decrease it in leo, but not WT zebrafish (F[3, 39] = 4.699, p = 0.0072). As in the novel tank test, freezing (F[3, 39] = 0.8265, NS; Figure I) did not differ between groups, nor did erratic swimming (H[df = 4] = 6.656, NS; Figure 2H). Risk assessment was greater in leo than WT, and fluoxetine decreased it in leo but not WT (H[df = 4] = 21.19, p < 0.001; Figure 2I). Tissue 5-HT levels were smaller in leo than in WT zebrafish (t[df = 18]= 3.093, p = 0.0063; Figure 3A), while 5-HIAA levels were similar (t[df = 18] = 0.8371, NS; Figure 3B). 5-HT turnover was higher in leo than WT zebrafish (t[df = 18] = 2.201, p = 0.041; Figure 3C). MAO activity was increased in leopard strain (t[df = 4] = 3.437, p = 0.0264; Figure 4). Discussion In the present article, we demonstrated that leo zebrafish mutants show a selective impairment in anxiety-like behavior in the light/dark preference (scototaxis) test and in the novel tank diving 9 (geotaxis) test. These tests have been shown to differ at the behavioral (Blaser & Peñalosa 2011, Blaser & Goldsteinholm 2012, Blaser & Rosemberg 2012, Luca & Gerlai 2012, Maximino et al. 2010, Wong et al. 2010) and pharmacological (Cachat et al. 2011, Egan et al. 2009, Maximino, Silva, Gouveia Jr & Herculano 2011, Maximino et al. In press) levels. In addition, we found that fluoxetine rescued the anxious phenotype of leo mutants in the light/dark test at a dose which was previously found to be inneffective in WT animals (Maximino et al. 2011, In press) and decreased measures of escape/avoidance in the novel tank test only in leo mutants. These results suggest that 5-HT transport is constitutively down-regulated in leo zebrafish. Consistent with this hypothesis, 5-HT tissue levels were lower in leo than WT zebrafish. While serotonin's metabolite, 5-HIAA, was not altered, 5-HT turnover was increased in leo mutants, consistent with the increased MAO activity in synaptosomal preparations from leo brains. These observations are somewhat similar to what is observed in Wistar-Zagreb rats selected for low platelet 5-HT uptake activity, which show decreased [3H]-citalopram binding in the brain and decreased 5-HT turnover (Cicin-Sain & Jernej 2010). However, this rat subline also shows decreased anxiety-like behavior. Likewise, spiegeldanio zebrafish mutants, which show reduced fibroblast growth factor receptor 1a function, show decreased anxiety-like behavior and decreased SERTa expression in the raphe nucleus; nonetheless, administration of fluoxetine did not rescure the anxiolytic-like phenotype, and zMAO expression was not different between spiegeldanio and WT animals (Norton et al. 2011; Norton & Bally-Cuif, 2012). The suggestion that this behavioral phenotype is mediated by different mechanisms than the anxious phenotype observed in the present work is supported by the observation that spiegeldanio decreased anxiety is reversed by treatment with manipulations of the histaminergic system (Norton et al. 2011). Recently, a mutation on the gene encoding the glucocorticoid receptor (grs357) has been shown to present increased startle responses as larvae (Griffiths et al., 2012) and exaggerated freezing responses in an open field as adults, as well as decreased SERTa expression in the raphe nucleus (Ziv et al., 2012); both phenotypes were rescued by 10 fluoxetine treatment. These conflicting results point to a complex regulation of behavioral phenotypes by serotonin transporters in zebrafish. The mutation which produces the leopard phenotype has been described as a missense mutation in the gene coding for a connexin, cx41.8 (Watanabe et al. 2006). This connexin is expressed in the central nervous system of zebrafish, but its cellular distribution is not known; nonetheless, hemichannels formed by connexins with the missense mutation show decreased coupling, which might decrease functional coupling between neurons and glial cells or glia-glia communication in the central nervous system. While serotonin transporters have been described in neurons and glial cells alike (Inazu et al. 2001, Pickel & Chan 2000), it is not clear whether the observations made herein point to a dysregulation of 5-HT uptake by glial networks (by, e.g., decreased metabolic coupling) or by neurons. Further studies are necessary to assess this hypothesis. Overall, the results presented herein suggest an important pathway associated with increased anxiety in different phenotypes. In addition to the pharmacological effects of 5-HTergic drugs in anxiety-like behavior (Maximino et al., In press), then, an important role for 5-HT in genetic differences in anxiety is also suggested for zebrafish. Thus, variations in 5-HTergic systems, especially uptake mechanisms, could represent an important target of genetic variation underlying anxiety disorders. Acknowledgments Part of this research was funded by CAPES/Brazil and by the Zebrafish Neuroscience Research Consortium. CM and BP are recipients of CAPES studentships. AMH is a recipient of a CNPq productivity grant. A previous version of this work was presented in poster format at the 1 st International Conference on Environmental Bioinorganic and Toxicology Research (CEBiTOR 2012). 11 Bibliography Aldeco M., Arslan B. K., Edmondson D. E. 2011, 'Catalytic and Inhibitor Binding Properties of Zebrafish Monoamine Oxidase (zMAO): Comparisons with Human MAO A and MAO B', Comparative Biochemistry and Physiology B, 159, pp. 78-83. Araújo J., Maximino C., Brito T.M. et al.. Behavioral and Pharmacological Aspects of Anxiety in the Light/Dark Preference Test. In Zebrafish Protocols for Neurobehavioral Research. Kalueff A.V. & Stewart A.M. (Eds.). 2012. pp. 191-202. Blaser R.E.& Peñalosa Y.M. 2011, 'Stimuli Affecting Zebrafish (Danio Rerio) Behavior in the Light/Dark Preference Test', Physiology & Behavior, 104, pp. 831-837. Blaser R.E.& Goldsteinholm K. 2012, 'Depth Preference in Zebrafish, Danio Rerio: Control by Surface and Substrate Cues', Animal Behaviour, 83, pp. 953-959. Blaser R.E.& Rosemberg D.B. 2012, 'Measures of Anxiety in Zebrafish (Danio Rerio): Dissociation of Black/White Preference and Novel Tank Test', PLoS ONE, 7, p. e36931. Cachat J., Stewart A., Utterback E. et al. 2011, 'Three-Dimensional Neurophenotyping of Adult Zebrafish Behavior', PLoS ONE, 6, p. e17597. Cicin-Sain L.& Jernej B.. Wistar-Zagred 5HT Rats: a Rodent Model With Constitutional Upregulation/Downregulation of Serotonin Transporter. In Experimental Models in Serotonin Transporter Research. Kalueff A.V. & LaPorte J.L. (Eds.). 2010. pp. 214-243. Dere E., De Souza-Silva M. A., Frisch C., Teubner B., Söhl G., Willecke K., Huston J. P., 2003. 'Connexin30-deficient Mice Show Increased Emotionality and Decreased Rearing Activity in the Openfield Along with Neurochemical Changes'. European Journal of Neuroscience, 18, pp. 629-638. Egan R.J., Bergner C.L., Hart P.C. et al. 2009, 'Understanding Behavioral and Physiological Phenotypes of Stress and Anxiety in Zebrafish', Behavioural Brain Research, 205, pp. 38-44. 12 Frisch C., Theis M., Silva M.A.D.S. et al. 2003, 'Mice With Astrocyte-Directed Inactivation of Connexin43 Exhibit Increased Exploratory Behaviour, Impaired Motor Capacities, and Changes in Brain Acetylcholine Levels', European Journal of Neuroscience, 18, pp. 2313-2318. Gilbert S.F. 2003, Developmental Biology, 7th, Sinauer Associates Inc. Griffiths B. B., Schoonheim P. J., Ziv L. et al. 2012. 'A Zebrafish Model of Glucocorticoid Resistance Shows Serotonergic Modulation of the Stress Response', Frontiers in Behavioral Neuroscience, 6, p. 68. Haffter P., Odenthal J., Mullis M. C. et al. 1996, 'Mutations affecting pigmentation and shape of the adult zebrafish', Development, Genes and Evolution, 206, pp. 260-276. Inazu M., Takeda H., Ikoshi H. et al. 2001, 'Pharmacological Characterization and Visualization of the Glial Serotonin Transporter', Neurochemistry International, 39, pp. 39-49. Kalueff A. V., Gebhardt M., Stewart A. M. et al. In press, 'Towards a comprehensive catalog of zebrafish behavior 1.0, and beyond', Zebrafish. Kinkel M. D., Eames S. C., Philipson L. H. & Prince V. E. 2010, 'Intraperitoneal Injection into Adult Zebrafish', Journal of Visualized Experiments, 42, p. 2126. Luca R.M.& Gerlai R. 2012, 'Animated Bird Silhouette Above the Tank: Acute Alcohol Diminishes Fear Responses in Zebrafish', Behavioural Brain Research, In press. Maximino C., 2012. Serotonin and Anxiety. Neuroanatomical, Pharmacological, and Functional Aspects. New York: Springer. Maximino C., Brito T.M., Colmanetti R. et al. 2010, 'Parametric Analyses of Anxiety in Zebrafish Scototaxis', Behavioural Brain Research, 210, pp. 1-7. Maximino C., Silva A.W.B., Gouveia Jr A. et al. 2011, 'Pharmacological Analysis of Zebrafish (Danio Rerio) Scototaxis', Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35, pp. 624631. 13 Maximino C., Puty B., Benzecry R. In press, 'Role of serotonin in zebrafish (Danio rerio) anxiety: Relationship with serotonin levels and effect of buspirone, WAY 100635, SB 224289, fluoxetine and para-chlorophenylalanine (pCPA) in two behavioral models', Neuropharmacology. Naus C.C.G., Bechberger J.F., Zhang Y. et al. 1997, 'Altered Gap Junctional Communication, Intercellular Signaling and Growth in Cultured Astrocytes Deficient In Connexin43', Journal of Neuroscience Research, 49, pp. 528-540. Norton W.H.J., Stumpenhorst K., Faus-Kessler T. et al. 2011, 'Modulation of Fgfr1a Signaling in Zebrafish Reveals a Genetic Basis for the Aggression-Boldness Syndrome', Journal of Neuroscience, 31, pp. 13796-13807. Norton W.H.J. & Bally-Cuif L. 2012, 'Unravelling the Proximate Causes of the Aggression-Boldness Behavioural Syndrome in Zebrafish'. Behaviour, 149, 1063-1079. Perry S. F., Davie P. S., Daxboeck C. et al., Perfusion Methods for the Study of Gill Physiology. In Fish Physiology Volume X: Gills, Part B: Ion and Water. Hoar W. S. & Randall D. J. (Eds). 1984. pp 325388. Pickel V.M.& Chan J. 2000, 'Ultrastructural Localization of the Serotonin Transporter in Limbic and Motor Compartments of the Nucleus Accumbens', Journal of Neuroscience, 19, pp. 7356-7366. Pinheiro S. H., Zangrossi Jr H., De-Ben C. M., Graeff F. G. 2007, 'Elevated Mazes as Animal Models of Anxiety: Effects of Serotonergic Agents', Anais da Academia Brasileira de Ciências, 79, pp. 71-85. Sáez J.C., Contreras J.E., Bukauskas F.F. et al. 2003a, 'Gap Junction Hemichannels in Astrocytes of the CNS', Acta Physiologica Scandinavica, 179, pp. 9-22. Sáez J.C., Berthoud V.M., Brañes M.C. et al. 2003b, 'Plasma Membrane Channels Formed by Connexins: Their Regulation and Functions', Physiological Reviews, 83, pp. 1359-1400. Stewart A. M., Cachat J., Green J. et al. 2013, 'Constructing the Habituome for Phenotype-Driven Zebrafish Research', Behavioural Brain Research, 236, pp. 110-117. 14 Weissbach H., Smith T.E., Daly J.W. et al. 1960, 'A Rapid Spectrophotometric Assay of Monoamine Oxidase Based on the Rate of Disappearance of Kynuramine', Journal of Biological Chemistry, 235, pp. 1160-1163. Wong K., Elegante M., Bartels B. et al. 2010, 'Analyzing Habituation Responses to Novelty in Zebrafish (Danio Rerio)', Behavioural Brain Research, 208, pp. 450-457. Watanabe M., Iwashita M., Ishii M. et al. 2006, 'Spot Pattern of Leopard Danio Is Caused by Mutation in the Zebrafish connexin41.8 Gene', EMBO Reports, 7, pp. 893-897. Ziv L., Muto A., Schoonheim P. J., et al. 2012. 'An Affective Disorder in Zebrafish With Mutation of the Glucocorticoid Receptor', Molecular Psychiatry Advanced online publication. Zor T., Selinger Z. 1996, 'Linearization of the Bradford Protein Assay Increases Its Sensitivity: Theoretical and Experimental Studies', Analytical Biochemistry, 236, pp. 302-308. 15 Figure legends Figure 1 – Differential effects of fluoxetine on WT and leotq270/tq270 zebrafish in the novel tank test. (A) Schematics of the experimental tank and its division for manual recording of behavioral events. (B-F) Effects of fluoxetine (0 or 5 mg/kg) on (B) bottom-dwelling, (C) habituation, (D) total locomotion, (E) erratic swimming, and (F) freezing in the novel tank test in leo (black bars, black boxplots, black full and empty circles) and WT (gray bars, gray boxplots, gray full and empty circles) zebrafish. **, p < 0.01. In B, C, and F, bars or circles represent mean ± S. E. M. In D and E, boxes represent data from the 25th to the 75th percentiles, the trace represents the median, the plus (+) sign the mean, and whiskers represent the 2.5th and 97.5th percentile. Figure 2 – Differential effects of fluoxetine on WT and leotq270/tq270 zebrafish in the scototaxis test. Effects of fluoxetine (0 or 5 mg/kg) on (A) white avoidance, (B) white avoidance habituation, (C) latency to white, (D) total locomotion on white, (E) entries on the white compartment, (F) locomotion habituation, (G) thigmotaxis, (H) freezing, (I) erratic swimming and (J) risk assessment in the light/dark tank in leo (black bars) and WT (gray bars) zebrafish. ***, p < 0.001; **, p < 0.01; *, p < 0.05; N. S., non-significant. In A, B, C, F, G, and H, bars represent mean ± S. E. M. In D, E, I and J, boxes represent data from the 25th to the 75th percentiles, the trace represents the median, the plus (+) sign the mean, and whiskers represent the 2.5th and 97.5th percentile. Figure 3 – Tissue levels of (A) 5-HT, (B) 5-HIAA, and (C) serotonin turnover (5-HIAA:5-HT ratio) of leo (black bars) and WT (gray bars) zebrafish. **, p < 0.01; *, p < 0.05. Bars represent mean ± S. E. M. Figure 4 – zMAO activity, measured as the rate of kynuramine disappearance in leo (black circles) and WT (gray circles) zebrafish. ***, p < 0.001; *, p < 0.05. Points represent mean ± S. E. M. 16