Spectra Lab
advertisement

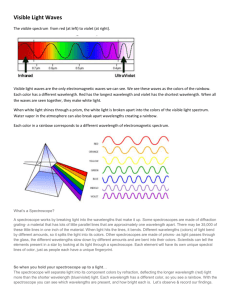
Chemistry Name:_________________ Emission Spectra of Gases Lab Procedure: Using a spectra-scope, observe the emission spectra of the following gases… Hydrogen (H), Helium (He), Argon (Ar), Mercury (Hg), Neon (Ne), Chlorine (Cl) All of these gases emit photons in the visible part of the electro-magnetic spectrum as well as in the infrared and ultraviolet range. However, the spectra-scope device we will be using is capable of seeing only those photons in the “visible” part of the electro-magnetic spectrum. You will find that each gas emits a variety of colors in the visible range, and in most cases you will see many shades of the same color. For example, Hydrogen emits photons of five different colors….two violets, one blue, and two reds. Very accurate spectra scopes can determine the exact wave lengths of each of the color. (Understanding star and nebula composition is simply a matter of comparison of star spectra to known gas spectra) Your spectra scope has the ability of giving you a “rough” estimate of wavelength for the various emitted colors. You will see the calibrated scale on your spectra scope as follows… So, from the data above, the light we are looking at has wavelengths of about…. 1 = 450 nm and 2 = 700 nm Observations: Hydrogen (H2 gas) 1. There are three colors that stand out the most, what are they? 2. How many red bands are visible? 3. For each of the three different colors, approximately what are their respective wavelengths (nm)? Helium (He gas) 4. Which five colors stand out the most? 5. How many different blue bands are visible? How about green bands? Yellow? Red? 6. About what wavelengths do the green and yellow lines have? Neon (Ne gas) 7. What four colors stand out the most? 8. How many different bands of yellow are visible? How about red bands? Argon (Ar gas) 9. Most of the colors are pretty weak, but what four colors stand out the most? 10. How many yellow bands are present? Green? Red? Mercury (Hg vapor) 11. How many violet bands are present? 12. About what wavelength do these violet bands have? 13. Are any orange bands present? (look hard) If so, about what wavelength does it have? 14. What are the wavelengths of the first and last red bands? Chlorine (Cl2 gas) 15. How many green bands stand out? 16. About what wavelengths do the violet bands have? ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ Questions: 17. Photons that have different wavelengths have what noticeable difference? 18. Describe in detail how an atom could emit photons of many different wavelengths? (use Bohr atom diagram to assist you) 19. How could spectroscopy be useful to an astronomer? 20. Which color has the longest wavelength? Which has the shortest? 21. For the green and yellow lines of Helium, determine the frequency () for each. 22. For the green and yellow lines of Helium, determine the Energy (in Joules) that each have. 23. For the first red band of Mercury, determine its frequency () and energy (E) in Joules.