Lab No
advertisement

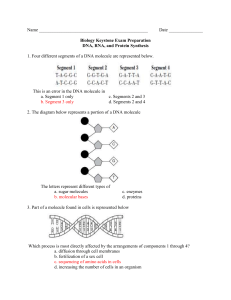
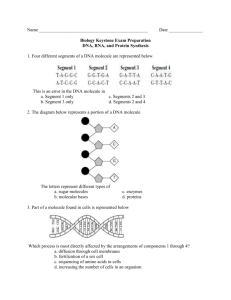
Lab No. _______________ Date __________________ Name_________________________ Biology Section _________________ THREE DIMENSIONAL MODELS OF CHEMICAL COMPONENTS AND REACTIONS IN LIVING THINGS ATOMS AND CHEMICAL BONDS Wooden balls of different colors represent different kinds of atoms according to the following key: carbon hydrogen oxygen nitrogen (C) (H) (O) (N) ----- black yellow red light blue For this exercise use springs and pegs as chemical bonds according to the following scheme: C–C C–O–C C–N use long wooden pegs C–H C–O–H N–H use short pegs C=O -- use springs The models of the atoms are drilled with one or more holes. Each hole represents an electron that may be shared with other atoms. The valence or combining power of an atom is represented by the number of holes. What is the valence of a hydrogen (H) atom? Oxygen (O)? 2. _____________________ 1. ___________________________ Carbon (C)? 3. ________________ Short, wooden pegs, long wooden pegs, and springs are used to connect the atoms in the formation of molecules of compounds and represent chemical bonds. Each chemical bond in the models represents a pair of electrons shared by two bonded atoms. This type of chemical bond is called a covalent bond. MODELS OF SIMPLE MOLECULES Using short pegs combine two hydrogen atoms to form a molecule of hydrogen (H2). A structural formula of a molecule is made by using chemical symbols for the atoms and a dash (-) for the connecting chemical bonds. Draw the structural formula for a molecule of hydrogen (H2). (4) Using springs (to help form double bonds) make a model of the oxygen molecule (O2). Draw the structural formula of a molecule of oxygen. (5) I-M 5/09 -2How many electrons are shared by the two oxygen atoms? (6) __________________________ Using short pegs make a model of a water (H2O) molecule and draw its structural formula. (7) Similarly make a model of carbon dioxide (CO2) using springs for chemical bonds. Draw the structural formula for carbon dioxide. (8) GROUPS AND SIMPLE ORGANIC MOLECULES Living things are composed of molecules that contain carbon atoms. Such molecules are said to be organic molecules as opposed to inorganic molecules that do not contain carbon. Construct a model of methane (CH4), the simplest organic compound. Write the structural formula for methane. (9) Remove a hydrogen from methane along with the bond to make a methyl (CH3) group. What is the valence of this group? (10) ___________________________ Now make an alcohol (OH) group. What is the valence of this group? (11)________________________ Combine the CH3 group with the OH group. The compound formed is methyl alcohol. Draw the structural formula for methyl alcohol and circle and label the alcohol group. (12) Make another CH3 group. Construct an amino (NH2) group (use only the holes in nitrogen that do not go clear through). Combine the methyl and the amino group for form methylamine. Draw the structural formula for methylamine and circle and label the amino group. (13) -3Construct an organic acid group (COOH). It should look like this: Connect the organic acid group to a CH3 group forming acetic acid. Draw the structural formula for acetic acid and circle and label the organic acid group. (Sometimes called the carboxyl group). (14) Construct an aldehyde group that looks like this: C-O-H O C–H O Connect the aldehyde group to a CH3 group forming acetaldehyde. Draw the structural formula for acetaldehyde and circle and label the aldehyde group. (15) CARBOHYDRATES Carbohydrates are composed of carbon, hydrogen and oxygen. The general formula for a carbohydrate is CnH2nOn. Note that the hydrogen to oxygen ratio is 2:1 as in water. There are twice as many hydrogens as carbons. A. MONOSACCHARIDES The simplest carbohydrates are the simple sugars called monosaccharides with the formula C6H12O6. The most commonly occurring simple sugar is glucose. Make a molecule of glucose as follows: C1 A. Assemble a chain of six carbon atoms as shown. C2 B. Make carbon #1 part of an aldehyde group. C3 C. Add an alcohol group to each of the other carbons. C4 D. Fill in any unsatisfied carbon valences with hydrogen. C5 E. Complete the linear diagram of glucose shown on the left. (16) C6 -4Put this molecule into a ring form (see diagram below) by twisting it to bring carbon #1 and carbon #5 close together. Remove one of the double bonds at carbon #1. In its places put an OH group which is taken from carbon #5. Complete bonding of oxygen by joining it to carbon #5. Complete the ring structure of glucose below. Recall that the formula for glucose is C6H12O6. (17) C6 C5 O C4 C1 C3 C2 B. DISACCHARIDES Cooperate with your neighbor and bring two models of glucose close together so that the OH group of carbon #1 of one molecule and the OH group of carbon #4 of the second molecule are next to one another. Remove one OH of one molecule and an H of the OH group of the second molecule. Join the H and the OH that have been removed. What molecule consists of an H join to an OH? (18) _________________ Now bond the carbon of one molecule (where the OH has been removed) to the O of the second model (where the H has been removed). The two remains of the glucose molecules have now been bonded together and form a new compound, a double sugar (disaccharide) known as maltose. Count the atoms in your model of maltose and write the molecular formula below. (19) Explain why this reaction that you simulated is called dehydration synthesis of two glucose molecules to form maltose. (20) The digestion of maltose is called hydrolysis. Simulate this reaction by splitting the water molecule into OH and H groups, breaking the bond that holds the two parts of maltose together, and adding the H and OH groups to reform the two molecules of glucose. The suffix “lysis” means to “break up”. Explain why the process of breaking up maltose is called hydrolysis. (21) -5Complete the equation below for dehydration synthesis and hydrolysis of maltose. (22) C6H12O6 + C6H12O6 dehydration synthesis hydrolysis C. POLYSACCHARIDES If we similarly joined all of the models of glucose prepared by this class, removing a molecule of water from between each molecule in the long chain, we would have a polysaccharide. Starch is such a molecule several thousand units long. Cellulose from which cell walls of plant cells are made is another long carbohydrate molecule. Both starch and cellulose can be hydrolyzed to glucose by the use of suitable enzymes. PROTEINS Proteins are made from small molecules called amino acids. Make a model of an amino acid as follows: A. Make a two carbon chain (C-C) B. Make one carbon an organic acid group: C-O-H O C. Add an amino group to the second C. N–H H Use only holes in the N that do not go all the way through the ball. D. Bond an H to the second carbon (the same one that is bonded to the amino group). The structure you have made is the fundamental part of any amino acid. Note that it has one remaining valence on the carbon atom to which the amino group is attached. There are 20 different kinds of amino acids each having a different group called the R group bonded to this remaining valence of carbon. Now construct a second model just like the first. -6Convert one of these models to the amino acid glycine by bonding an H to the empty carbon valence. The H is the R group of glycine. Convert the second model to alanine by bonding a CH3 to the empty carbon valence. The CH3 group is the R group for alanine. Complete the structural formula for each of the models of amino acids that you have made. (23) After you have completed the formula, circle and label the R group, organic acid group (carboxyl group), and the amino group. (24) Lay the two amino acids down so that the amino groups of one is next to the organic acid group of the other as below. Remove an OH group from the organic acid group of one amino acid and an H from the amino group of the other amino acid. Join the H and OH. What model have you made? (25) -7Bond the remains of the two amino acids by joining the carbon of one to the N of the other. This C-N bond is called a peptide bond. Circle this bond in the diagram below. The new molecule formed is a dipeptide. Complete the structure below. (26) N–C–C–N–C–C If you joined many amino acids together, a polypeptide would be formed. Join your dipeptide to your neighbors’ forming a polypeptide with four units. Most proteins are long polypeptide chains consisting of several hundred amino acids. If you separated the units of the chain by adding water in the proper places, you would reform the amino acids. When you do this, you are simulating the process of hydrolysis or digestion of proteins. LIPIDS Lipids are the group of organic compounds that include: fats, oils, waxes and sterols. Fats are large molecules that contain carbon, hydrogen and oxygen although in different ratios than carbohydrates. All fats contain three fatty acid molecules combined with a molecule of glycerol. H H H H–C –C – C–H OH – OH – OH glycerol H H H O H – C – C ….... C – C – O – H H–H a fatty acid H -8What type of compound is glycerol? (Hint: What group do you recognize?) (27) What makes the fatty acid shown above an acid? (28) In the synthesis of a “fat”, glycerol and three fatty acids react in the following way: How many water molecules are formed in the above reaction? (29) What reaction would occur when this fat molecule is digested back into glycerol and fatty acids? (30)