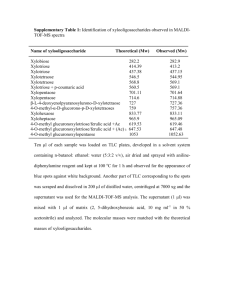
Purification of His-p53 (following protocol of Shieh et al
advertisement

Purification of His-p53 7/15/03 1. Inoculate a 60 ml LB (100 g/ml ampicillin; 60 l 100 mg/ml stock) culture with BL21 E. coli with His-p53 -Incubate at 37oC overnight 2. Dilute overnight culture 1:10 with LB plus 100 g/ml ampicillin (60 ml overnight culture with 540 ml LB) -Take an OD600 reading of cells -Put cells at 37oC until grown to an OD600 of 0.5 3. Add IPTG to a final concentration of 1mM (6 ml 100 mM stock) and incubate at 25oC for 2 hours to induce His-p53 expression 4. Pellet cells: Spin at 6,000xg for 15 minutes at 4oC (split culture in four 250 ml bottles) 5. Remove supernatant, resuspend two pellets in 15 mls sonication buffer (0.3 M NaCl, 50 mM NaH2PO4, pH 8.0, 20% glycerol, 1% NP-40, 10 mM mercaptoethanol, 0.5 mM PMSF) and transfer to two 40 ml plastic tubes 6. Freeze pellets in a dry ice/ethanol bath, thaw on ice 7. Sonicate samples : 10 seconds on, 10 seconds off for a total of 2 minutes 8. Pellet cellular debris 10,000 xg for 10 minutes at 4oC -SAVE 200 l of total lysate for SDS-PAGE analysis 9. Transfer supernatant to a new 50 ml conical tube containing prewashed Ni-NTA resin, incubate at 4oC for 60 minutes with rocking -Preparation of resin: Mix 50% slurry and transfer 1 ml to a 50 ml conical tube, add 5 ml of sonication buffer and mix, once resin settles remove supernatant 10. Pellet resin in a tabletop centrifuge, 1,200rpm for 3 minutes at 4oC -Carefully remove supernatant with the vacuum 11. Wash resin with 25 mls of sonication buffer minus NP-40 plus 40 mM imidazole -Spin at 1,200 rpm for 5 minutes at 4oC, carefully remove supernatant and wash a second time 12. Elution: Add 1 ml of sonication buffer minus NP-40 plus 0.25 M imizadole to resin -Incubate at 4oC for 15 minutes with rocking to elute His-p53 -Spin at 1,200 rpm for 5 minutes at 4oC -Carefully remove supernatant and transfer to an eppendorf tube (fraction 1) -Repeat to obtain fraction 2 -SAVE 40 l of fraction 1 and 2 for SDS-PAGE analysis 13. Mix fractions 1 and 2 -Put into a dialysis cassette and place in 500 mls kinase buffer (50 mM Tris, pH 7.5, 10 mM MgCl2, 1mM DTT), put at 4oC ON 14. Remove dialysis cassette and use syringe to collect His-p53 15. Make 200 l aliquots and freeze at –80oC -SAVE 40 l for SDS-PAGE analysis