07 Finding Oxidation Numbers Worksheet
advertisement
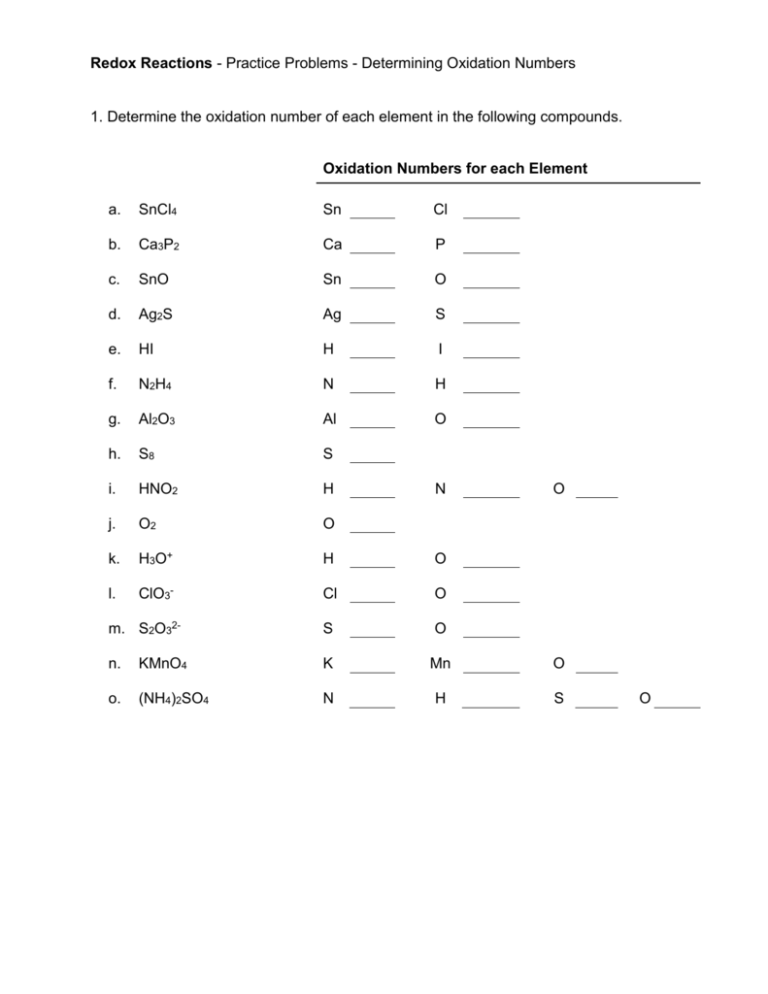
Redox Reactions - Practice Problems - Determining Oxidation Numbers 1. Determine the oxidation number of each element in the following compounds. Oxidation Numbers for each Element a. SnCl4 Sn Cl b. Ca3P2 Ca P c. SnO Sn O d. Ag2S Ag S e. HI H I f. N2H4 N H g. Al2O3 Al O h. S8 S i. HNO2 H j. O2 O k. H3O+ H O l. ClO3- Cl O m. S2O32- S O n. KMnO4 K Mn O o. (NH4)2SO4 N H S N O O 2. Determine the oxidation number of carbon in each of the following compounds: a. methane, CH4 b. formaldehyde, CH2O c. carbon monoxide, CO d. carbon dioxide, CO2 Redox Reactions - Practice Problems - Determining Oxidation Numbers - KEY Source: http://www.saskschools.ca/curr_content/chem30_05/6_redox/practice/redox_practice1.htm 1. Determine the oxidation number of each element in the following compounds. Oxidation Numbers for each Element a. SnCl4 Sn +4 Cl -1 b. Ca3P2 Ca +2 P -3 c. SnO Sn +2 O -2 d. Ag2S Ag +1 S -2 e. HI H +1 I -1 f. N2H4 N -2 H +1 g. Al2O3 Al +3 O -2 h. S8 S 0 i. HNO2 H +1 N +3 j. O2 O 0 k. H3O+ H +1 O -2 l. ClO3- Cl +5 O -2 m. S2O32- S +2 O -2 n. KMnO4 K +1 Mn +7 O -2 o. (NH4)2SO4 N -3 H +1 S +6 watch the sign for N! O -2 pure element! surprised? O -2 Extra help for KMnO4 & (NH4)2SO4 You will need to recognize polyatomic ions. It will simplify determining oxidation numbers to break molecules with polyatomic ions into two separate ions, then find oxidation numbers for each part separately. KMnO4 K+ Oxidation Number = MnO4- oxygen’s oxidation number = -2 sum of ox. nos. for MnO4therefore oxidation number of Mn 4 oxygens total = Hydrogen’s oxidation number = +1 charge of ammonium ion 4 hydrogens total = (NH4)2SO4 NH4+ +1 -8 -1 +7 +4 +1 therefore oxidation number of N -3 + Note – there are two NH4 ions, but the oxidation numbers in both will be the same! oxygen’s oxidation number = -2 charge of sulfate ion SO42- 4 oxygens total = -8 -2 therefore oxidation number of S +6 2. Determine the oxidation number of carbon in each of the following compounds: a. methane, CH4 C = -4 element H C Ox. No. +1 -4 b. formaldehyde, CH2O C=0 No. Total Atoms 4 +4 1 -4 SUM 0 element H O C Ox. No. +1 -2 0 No. Atoms 2 1 1 SUM c. carbon monoxide, CO C = +2 element O C Ox. No. -2 +2 No. Total Atoms 1 -2 1 +2 SUM 0 Total +2 -2 0 0 d. carbon dioxide, CO2 C = +4 element O C Ox. No. -2 +4 No. Atoms 2 1 SUM Total -4 +4 0







