Chemistry Lab Techniques
advertisement

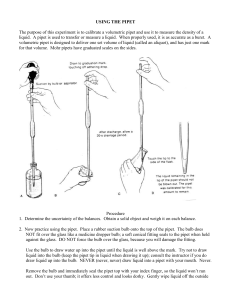
Chemistry Lab Techniques Measuring the volume of a liquid with a graduated cylindar: The surface of a liquid confined in a cylindar curves to form what is known as a meniscus. The meniscus of most liquids curves up the sides of the container, making the center of the curve appear lower than the edges. Since reading the meniscus at the top or at the bottom of the curve will make a difference in the volume measured, it is generally agreed to always read the bottom of the curve. The smaller the container, the greater the curve of the meniscus. As the diameter of the cylindar increases, the curve of the meniscus flattens out. By holding the card behind the cylindar, and immediately below the bottom of the meniscus, the volume can be easily read. Measuring the volume of a liquid with a pipet: Pipets are much more accurate than graduated cylindars. Reading the volume of liquid in a pipet is just like reading a graduated cylindar, however there is one additional technique needed with a pipet. The diameter does not allow a liquid to be poured into a pipet - the liquid must be drawn into the pipet. This picture shows two of several types of pipet bulbs used to draw a liquid into a pipet. The red bulb is known as a standard pipet bulb. The black bulb is known as a safety pipet bulb. Much of the "professional" lab work today is done with automatic pipets. These are expensive little gadgets that come in many different volumes, each delivering exactly its assigned volume with one click of a button. High school chemistry students need to know the basic techniques of pipetting, so you will be using glass pipets and the standard pipet bulb. The standard pipet bulb requires manual dexterity that is improved by repetitive use. You may have to practice using the bulb with a pipet before you are able to accurately transfer a measured volume of liquid. The technique goes something like this: Pour slightly more liquid than needed into a beaker using the "ballpark" graduations on the beaker. Never pipet directly from a reagent bottle. Place the tip of the pipet below the surface of the liquid in the beaker. Squeeze the pipet bulb and press it firmly over the top of the pipet. Do not force the bulb onto the pipet. The soft plastic collar of the bulb is tapered inside to insure a good seal as long as you keep a steady pressure between the pipet and the bulb. Gradually release the presure of your squeeze on the bulb and allow the liquid to be drawn into the pipet. Draw more liquid than needed, but do not allow the liquid to enter the bulb. With the tip of the pipet still below the liquid's surface, quickly remove the bulb and place your finger or thumb over the top of the pipet to prevent the solution from draining back into the container. Record the exact volume of liquid in the pipet (remember the meniscus). (Note: this is the step that requires the most practice). To release the liquid, slowly roll your finger to the side just enough to break the seal over the top of the pipet and allow the liquid to flow. To stop the flow of liquid, roll your finger back Measuring mass with a triple-beam balance: The triple-beam balance was once the "standard" balance in the general chemistry lab. While the electronic balance has replaced it in many cases, good science students should be familiar with the triple-beam balance and how to read one. The balance is named for its three "beams". An object is placed on the pan of the balance and tares on the beams are moved to balance the mass. As you face the balance, the back beam is graduated in 10 gram steps and the middle beam is graduated in 100 gram steps. It is very important that the tares on these two beams are in the notch for the whole number of grams and not in between notches. The front beam is a sliding scale graduated in grams. The tare on this beam can be positioned anywhere on the scale. Masses on a triple-beam balance can be read to tenths of a gram, and estimated to hundredths. Using a laboratory burner: Many chemistry experiments require something to be heated. This is done with one of several types of laboratory burners. The lab burners at Howe High School use propane gas delivered through the gas outlets at student lab stations. Before attempting to light any lab burner, check to see that the jet hole between the base and the burner tube is free of obstruction. If chemicals have covered this jet, the burner will not operate properly. After attaching the hose to the gas outlet, turn the handle on the outlet parallel to the nozzle to open the gas valve. The gas valve is turned off by turning the handle 90 degrees in either direction. Carefully check to see that you hear gas escaping from the mouth of the burner tube. When you are sure that you have gas, bring the head of the striker over the burner and squeeze the striker handle. The spark produced will ignite the gas and your burner is lit. Adjust the air control vent so that the flame has the proper color pictured here. A yellow flame is an indication of a lack of oxygen, meaning that the air vent needs to be opened. The hottest part of the burner flame is just at the top of the bright blue inner cone. Normal heating is done with an object at the top of the light blue outer cone, while strong heating is done with an object at the top of the bright blue inner cone. To heat a container gently, move the container back and forth through the outer cone. Filtering a precipitate from a solution: Filtering a solid out of a liquid is done using filter paper and a filter funnel. The filter funnel is supported by the ring on a ring stand. Lay a clay triangle across the ring, then place the filter funnel into the triangle. To prepare the filter paper, fold the paper in half, then fold it in half again. When you look at the open edge of the folded paper you will see four edges of paper. With thumb and finger, catch three of these edges. Squeeze the sides of the folded paper and a cone will form with three thicknesses of paper on one side and one thickness of paper on the other. Place this cone of paper into the filter funnel. Place a "catch container" under the stem of the filter funnel and adjust the height of the ring on the ring stand until the tip of the stem is below the mouth of the container. Use a wash bottle and wet down the inside of the filter paper. This will help it stick to the funnel. You are now ready to filter. Carefully pour the liquid to be filtered into the mouth of the funnel. Do not let the liquid rise to the top of the filter paper. If any liquid goes over and around the paper, your procedure is ruined. Be patient, it will take time for the liquid to move through the pores of the paper. When all the original liquid has been poured into the funnel, use the wash bottle to rinse any remaining precipitate out of the original container. Do not touch or try to stir the liquid inside the filter paper. The wet paper is easily torn, which will ruin your procedure. If the objective of your filtration is the solid-free liquid, throw the filter paper and its contents into the trash. If your objective is the solid, carefully remove the filter paper and set it in a secure place to dry.