Basic Laboratory Techniques
advertisement

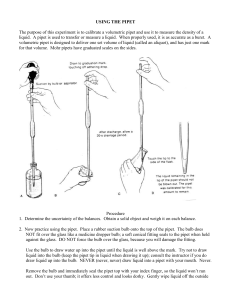
LAB EXERCISE: Basic Laboratory Techniques Introduction Scientists use measurements in describing objects and these measurements are based on universally accepted standards. A measurement of height specifies exactly how tall something is, whereas words such as “tall” or “short” are open to a wide range of interpretations. In the scientific community, standardized measurements (length, weight and volume) are fundamental to communication. The metric system of measurement is used for all measurement in most countries of the world. The scientific community of the entire world expresses data using the metric system. Therefore, it is necessary for us to know and to be able to effectively use this measurement system. It is also necessary to be able to convert English units into metric units. The metric system units are related to each other by a factor of ten, so interconversions are done by simply moving the decimal point to the left or right. The standard unit of length is the meter (m). The standard unit of mass is the gram (g). The standard unit of volume is the liter (L). For measuring time, the second, minute and hour are units that are used. Names of multiples or fractions of Metric units are formed by adding a prefix to “meter”, “gram” or “liter.” Prefix Symbol Meaning Base Unit Multiplied by teraT trillion 1,000,000,000,000 gigag billion 1,000,000,000 megaM million 1,000,000 kilok thousand 1,000 hectoh hundred 100 decada ten 10 single units, no prefix - Examples: meter, liter, gram 100 decid tenth 0.1 centic hundredth 0.01 millim thousandth 0.001 microu millionth 0.000001 nanon billionth 0.000000001 picop trillionth 0.00000000001 Factor 1012 109 106 103 102 101 10-1 10-2 10-3 10-6 10-9 10-12 VOLUME:WEIGHT CORRELATION: A milliliter is 1/1000 of a liter. This is approximately equal to 1 cubic centimeter (1 cc or 1 cm3). Furthermore, 1 ml of pure water weighs 1 gram at standard temperature and atmospheric pressure. This is a very convenient conversion (volume : weight) that you should know (i.e. 1 ml water = 1 gram water and 1 l water = 1 mg water). Measuring the volume of a liquid with a graduated cylinder: The surface of a liquid confined in a cylinder curves to form what is known as a meniscus. The meniscus of most liquids curves up the sides of the container, making the center of the curve appear lower than the edges. Since reading the meniscus at the top or at the bottom of the curve will make a difference in the volume measured, it is generally agreed to always read the bottom of the curve. The smaller the container, the greater the curve of the meniscus. The figure to the left is the meniscus in a 10 mL graduated cylinder. Measuring the volume of a liquid with a pipet: Pipets are much more accurate than graduated cylinders. Reading the volume of liquid in a pipet is just like reading a graduated cylinder, however there is one additional technique needed with a pipet. The diameter does not allow a liquid to be poured into a pipet - the liquid must be drawn into the pipet. This picture shows standard pipet pumps used to draw a liquid into a pipet. You may have to practice using the pump with a pipet before you are able to accurately transfer a measured volume of liquid. How to use a pipet pump: • Pour slightly more liquid than needed into a beaker using the "ballpark" graduations on the beaker. Never pipet directly from a reagent bottle. • Gently twist the pipet pump and press it firmly over the top of the pipet. Do not force the pump onto the pipet! You may break the glass pipet. • Place the tip of the pipet below the surface of the liquid in the beaker. • Slowly, draw in more liquid than needed, but do not allow the liquid to enter the pump. Graduated pipets come in a variety of styles: glass, plastic, reusable, disposable, marked for complete delivery and marked for delivery of a specific volume (i.e. you do not fully expel the solution from the pipet). Measuring mass with an electronic balance: The electronic balance has many advantages over other types of balance. The most obvious is the ease with which a measurement is obtained. All that is needed is to place an object on the balance pan and the measurement can be read on the display to hundredths of a gram. A second advantage, using the Zero button on the front of the balance, is less recognized by beginning science students. Because one must never place a chemical directly on the balance pan, some container must be used. Place the container on the balance and the mass of the container will be displayed. By pressing the Zero button at this point, the balance will reset to zero and ignore the mass of the container. You may now place the substance to be weighed into the container and the balance will show only the mass of the substance. This saves calculation time and effort. However, when the container is removed from the balance, the display will go into negative numbers until the Zero button is pressed again. Accuracy and Precision: The accuracy of an analytical measurement is how close a result comes to the true value. Accuracy refers to the agreement between a measurement and the true or correct value. Determining the accuracy of a measurement usually requires calibration of the analytical method with a known standard. Precision is the reproducibility of multiple measurements and is usually described by the standard deviation, standard error, or confidence interval. Precision refers to the repeatability of measurement. Statistical Significance: Tests of Significance allow biologists to estimate the probability that any differences between their experimental results and the predictions of the null hypothesis are due to chance alone. This probability is abbreviated as P. Usually, biologists accept a level of uncertainty of 5% or less (P < 0.05). A P = 0.05 means that at most (estimated) a 5% chance that you will be wrong if you reject the null hypothesis (the hypothesized value of the parameter is called the null hypothesis). A difference between the observed results and those predicted under the null hypothesis that is large enough to produce such a small P value is termed a significant difference. Confidence Intervals: A confidence interval gives an estimated range of values which is likely to include an unknown population parameter, the estimated range being calculated from a given set of sample data. If independent samples are taken repeatedly from the same population, and a confidence interval calculated for each sample, then a certain percentage (confidence level) of the intervals will include the unknown population parameter . Confidence intervals are usually calculated so that this percentage is 95%, but we can produce 90%, 99%, 99.9%, confidence intervals for the unknown parameter. The width of the confidence interval gives us some idea about how uncertain we are about the unknown parameter (see precision). A very wide interval may indicate that more data should be collected before anything very definite can be said about the parameter. Confidence intervals can be more informative than the simple results of hypothesis tests (where we decide "reject H0" or "don't reject H0") since they provide a range of plausible values for the unknown parameter. Graphing: Scientists always want to present their results with the least amount of error. There are many ways to help reduce error. One way is to average your results. This means to take all of your results and add them together and then divide by the number of things that you added; if you measured something five times, then add the five measurements together and divide by 5. Another way to help reduce the error is by plotting the results on a graph and then drawing a best fit line or curve. When drawing a best fit line, plot the data points and then place your ruler along them. Move your ruler around until you get about the same number of points above and below the ruler edge remembering to guide by the first and last points. Once you have positioned the ruler, use it to draw your line. Some of the points may land on the line while others may be scattered far away. You may not get any on the line at all. This line shows where the points would have been if there had been no error. The closer the points are to the line, the more accurate they are. Best Fit Line --- = Correct --- = Incorrect Not everything can be plotted in a linear graph. Many measurements in astronomy have graphs that curve up and down. In these cases you need to draw a best fit curve. Plot your data points and notice how the dots tend to suggest a direction for the curve. DO NOT connect-the-dots when drawing the curve! Remember, we are trying to get rid of the error. Instead, draw a freehand smooth curve that follows the general trend of the points. Best Fit Curve --- = Correct --- = Incorrect Name: __________________________ Basic Laboratory Techniques Exercises 1. Complete the following conversions: 0.543 kilograms is equivalent to ________________ grams 1271.72 liters is equivalent to ________________ milliliters 4123.43 centimeters is equivalent to ________________ meters 72.3 milligrams is equivalent to ________________ micrograms 169.3 microliters is equivalent to ________________ milliliters 200 micrometers is equivalent to ________________ centimeters 2. What is the volume of water as seen in the figure for “Measuring the volume of a liquid with a graduated cylinder” ? 3. Each group has been provided five pieces of string. a) Measure the length of each piece. b) Convert measurement into metric values. c) Weigh each piece. d) Graph length (cm) vs. weight (g) e) Graph length (m) vs. weight (mg) How do the two graphs (graph for d and graph for e) compare to one another? Table 1: Measured values of individual pieces of string. Piece # Length (in) Length (cm) Length (m) Weight (g) Weight (mg) 1 2 3 4 5 d) Graph your results using a line graph. Properly label the x and y axis. 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 0 0.5 1 1.5 2 2.5 Figure 1. _______________________________________________________________ ________________________________________________________________________ e) Graph your results using a line graph. Label the x and y axis. 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 0 0.5 1 1.5 2 2.5 Figure 2. _______________________________________________________________ ________________________________________________________________________ 4. a) Practice pipeting 10ml of water, using both a 5ml pipet and a 10ml pipet. b) Weigh out 10ml of water using 10ml pipet, five times. Calculate mean, deviation and squared deviation. Record data in the table below. 10ml pipet Trial #1 Trial #2 Trial #3 Trial #4 Trial #5 (g) (g) (g) (g) (g) Sum Water Weight Deviation (Data- mean = d) Squared Deviation (d 2) Record your mean (sum of water weight for all trials/number of trials): _____________________________ c) Weigh out 10ml of water using 5ml pipet, five times. Calculate mean, deviation and squared deviation. Record data in the table below. 5ml pipet Trial #1 Trial #2 Trial #3 Trial #4 Trial #5 (g) (g) (g) (g) (g) Water Weight Deviation (Data- mean = d) Squared Deviation (d 2) Record your mean (sum of water weight for all trials/number of trials): _____________________________ Sum d) Calculate standard deviation, standard error (SE), confidence interval (CI), upper and lower confidence limits for both 10ml and 5ml trials. Record your calculations in the table below. 10ml pipet 5ml pipet Stdev = sqrt (sum d2 / ( n-1)) SE = sd / sqrt ( n) CI = 2*SE UCL = mean + CI LCL = mean - CI e) Graph your results. Label the x and y axis. 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 0 0.5 1 1.5 2 2.5 Figure 3. _______________________________________________________________ ________________________________________________________________________