Paper
advertisement

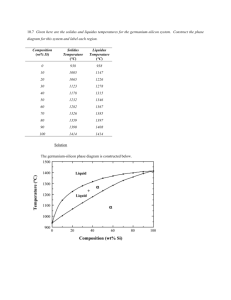
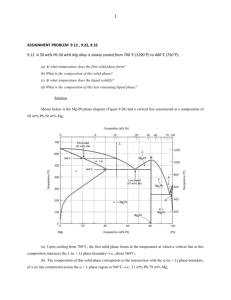
A PHASE FIELD MODEL FOR THE SOLIDIFICATION OF MULTICOMPONENT ALLOYS Pil-Ryung Cha, Dong-Hee Yeon and Jong-Kyu Yoon School of Materials Science and Engineering, Seoul National University, Seoul 151-742, Korea ABSTRACT A recently developed phase field model for the isothermal solidification of multicomponent alloys is presented. Using this model, the 2-D dendritic growth of binary and ternary alloys is simulated. The existing phase field model for the solidification of alloys is constrained to binary alloy systems. Particularly, when the solute atom is interstitial, the existing phase field model is not applicable. In this study, it will be shown that the existing phase field model for the isothermal solidification of binary alloys is not applicable to alloy systems with interstitial alloy elements and that our model for the solidification of multicomponent alloys could be applied to any alloy system with both interstitial and substitutional elements. 1.INTRODUCTION Understanding dendritic solidification is of great importance as the microstructural scales of dendrites control the segregation pattern and consequently determine the properties of the materials. Although there have been significant developments in understanding dendritic structures in past decades, our knowledge on the dendritic growth phenomena is largely based on experiments and idealized theorectical models. Therefore, the comprehensive description of dendritic solidification still remains a formidable task. Recently, the phase field model has been proposed to simulate solidification of pure materials[1-3] and binary alloys[4-8]. The phase field model is a very useful method for realistically simulating microstructure evolution with which diffusion in the solid and liquid phases, coarsening of dendrites and the curvature and kinetics effect on the moving S/L interface can be described. This model is very efficient, especially in numerical treatment, because all governing equations are written in unified forms without distinguishing the interface from solid and liquid phases. In the model, the phase field ( x, t ) characterizes the physical state( 1 in the solid, 0 in the liquid and 0 1 at the interface) of the system at each position and time. The phase field variable changes steeply but smoothly at the S/L interface region, which avoids direct tracking of the interface position. 282 The proposed models for alloy solidification[4-8] are restricted to binary alloys. Particularly, when the solute atom is interstitial, these models are not applicable[9,10]. The application of the phase field model for solidification to practical technology is closely linked to our ability to model microstructural development in multicomponent alloys (three or more components). Therefore, it is very important to develop a phase field model for the solidification of multicomponent alloy systems with both substitutional and interstitial elements. In this study, a phase field model is proposed for the solidification of multicomponent alloys applicable to both substitutional and interstitial alloys with thin interface limit. Our model was developed based on the WBM model[4] for the solidification of binary alloys and has the limitation for the interface thickness such as the WBM model for binary alloys. The detailed derivation of this model can be found in Ref.[10]. The previous work[9] of authors is the development of the phase field model for the solidification of multicomponent alloys based on the model proposed by Kim, et al.[7] and this model[9] has no limitation on the interface thickness. 2.DEVELOPMENT OF MULTICOMPONENT ALLOY PHASE FIELD MODEL We consider an isothermal solution with n alloy elements which may exist as either a solid or a liquid in a domain, . We choose the mole number per unit volume as the concentration variable. It is assumed that (i) the difference in the molar volume between the solid and liquid phase is negligible, (ii) substitutional elements and the solvent have the same molar volume( V S ), (iii) interstitial elements do not contribute to the entire volume, that is, the molar volume of interstitial elements is negligible, and (iv) the effect of substitutional vacancys is also negligible. Because the mole number per unit volume is used as the concentration variable, the two following constraints are imposed. 1 ci , (1) VS iS and c iI i cVa const. , (2) where the symbols S and I indicate the substitutional and interstitial element, respectively and , c i and cVa are the mole number of the i-th component and the mole number of vacancies per unit volume in the interstitial sites, respectively. From Eqs. (1) and (2), the concentrations of the solvent and interstitial vacancies are removed from concentration variables, where the solvent element is the n-th element. Consequently, the number of concentration variables is n-1. The analysis begins based on the formulation through the free energy functional of 283 Wheeler,Boettinger and McFadden[4]. The total free energy of the system is defined as F , c1 ,, cn1 f ( , c1 ,, cn1 ) 2 2 d , 2 (3) where f is the free energy density of the system and is a phase field taking a value of zero in the liquid and smoothly changing to one in the solid. We require that f (1, c1 ,, cn1 ) and f (0, c1 ,, cn1 ) are the the thermodynamic free energy densities for homogeneous solid and liquid. In order to derive a kinetic model, it is assumed that the system evolves in time so that its total free-energy decreases montonically. Since concentration is the conserved field variable and phase field variable is not conserved, the composition field is determined by solving the Cahn-Hilliard diffusion equation[11], whereas the phase field variable is determined by solving the Ginzburg-Landau equation[12]. This case corresponds to Model C of Hohenberg and Halperin[13]. The evolution equation for phase field is M 2 2 f , t (4) and the evolution equation for the k-th concentration variable is n 1 ck M ki f ci t i 1 n 1 n 1 n 1 j 1 i 1 i 1 M ki f cic j c j M ki f ci , (5) n 1 where M is the phase-field mobility and M ki is determined using Dkj M ki f ci c j . i 1 The phase field equation (4) and the diffusion equation (5) can be derived in a thermodynamically consistent way from the entropy functional. In this study, the free energy density of the system is defined as follows: f (c1 ,, cn1 ) h( ) f S (c1 ,, cn1 ) (1 h( )) f L (c1 ,, cn1 ) wg ( ), (6) where g ( ) is a double-well potential and w is the height of the double-well potential. The parameters and w in the phase field equation are to be matched with the interface energy and interface thickness 2 . The relationships among , w , and 2 are as follows[10]. 1 2 (0 ) (0)d0 , (7) 0 2 2 d 0 0.9 0.1 ( 0 ) (0) 284 . (8) n 1 where (c1 ,, cn1 , ) f (c1 ,, cn1 , ) f cei ci , 0 is phase field profile at the i 1 equilibrium state, f cei is the equilibrium chemical potential of i-th component, and Z (0 ) is defined by n 1 Z ( 0 ) h( 0 ) f S (c~1 ( 0 ),, c~n1 ( 0 )) 1 h( 0 ) f L (c~1 ( 0 ),, c~n1 ( 0 )) f cei c~i . (9) i 1 where c~i is the concentration profile of i-th component in an equilibrium state. The detailed derivation of the above relationships can be found in Ref.[10] In order to determine the phase field mobility at the thin interface thickness condition and low velocity limit, the growth velocity that chemical rate theory yields will be used[14]. The chemical rate theory for one componental systems describes interface movement by assuming thermally activating individual hopping processes of atoms across the interface. The theory yields for the growth velocity, G (10) V V0 (T ) 1 exp , RT is the maximal growth velocity when the driving force is infinite (no where V0 backward hopping) and G is the driving force per mole( F / ctot , where ctot is the total moles of elements per unit volume) for the solidification. In the low velocity limit, i.e., V V0 , by a Taylor series expansion, Eq.(10) becomes approximately V V0 FS / ctot G V0 , RT RT (11) where ctot is the total moles of elements per unit volume and 1/ VS iI ci . The driving force for the solidification without the solute-drag effect in a multicomponent system is given by[9] FS f (c ,, c S S 1 S n 1 ) f ( c , , c L L 1 n 1 L n 1 ) j 1 f L c c . c Lj L j L j (12) Using Eq. (11), the phase field mobility will be determined. Under the assumption of negligible diffusivity in solid, we will derive the corresponding relationship for the present multicomponent alloy phase field model at a thin interface limit condition. At a 1-D instantaneous steady state with an interfacial velocity, V, the governing equations for concentrations become dck d n1 d M ki f ci . (13) dx dx i 1 dx The following equation can be derived from Eq. (13) to the first order in the Peclet V 285 ~ ~ number, P 2V / D ( D : average interface diffusivity)[10], n 1 f L (c1 ,, cn1 ) f S (c1 ,, cn1 ) ciL ciS f cLi V , (14) i 1 where is a constant for a given temperature and is defined as follows[10]: 1 M n 1 c L ,e n 1 j d0 dx B ejk ck0 ckS ,e dxdc j , S ,e dx cj x j 1 k 1 2 (15) where ck0 and 0 are the k-th composition and phase field at an equilibrium state, respectively. It should be noted that the left hand side of Eq.(14) is the same as the driving force for the solidification per unit volume. So, using Eq.(11) and Eq.(12), the phase field mobility in the finite interface limit can be determined from the following equation RTc tot 0.1 n1 n1 (16) ji f ci0 j (0 )d0 , V0 M 2 2 0.9 j 1 i 1 where ctot 1/ VS ciS ,e , the matrix, , is the inverse matrix of , ij is iI defined as f cic j and j (0 ) 0.1 n 1 0 k 1 B ejk ck0 ckS ,e d . ( ) (0) 3.NUMERICAL SIMULATION In order to verify multicomponent alloy phase field model, numerical simulations for the isothermal solidification of ternary alloy systems are presented. Based on the verified phase field model for multicomponent alloys, 2D dendritic growth of binary and ternary alloys with interstitial alloy elements will be presented. 3.1 Validation of the model In order to confirm the validity of our model, the solidification of ternary alloy systems is considered. We selected an alloy system with only substitutional alloy elements, FeCr-Ni and an alloy system with both substitutional and interstitial alloy elements, FeMn-C for the numerical simulation. The verification of our model will be conducted from the two viewpoints. One is whether our model evolves from the non-equilibrium state to the equilibrium state and produces the equilibrium volume fraction and concentrations of solid and liquid phases. The other is whether our model realizes the kinetics of solidification. First, it will be shown that our model can produce exactly the equilibrium state of 286 solidification. The Fe-9.0at%Cr-10.0at%Ni alloy system solidifies from liquid to austenite(FCC) at 1757K and the Fe-1.0at%Mn-0.5at%C alloy system solidifies from liquid to -ferrite(BCC) at 1780K. The surface excess energy, , was set at 0.4J/m2, and then, the limitation of the interface thickness[10] gives the condition of 2 0.973m in the Fe-9.0at%Cr-10.0at%Ni alloy system at 1757 K and 2 0.085m in the Fe-1.0at%Mn-0.5at%C alloy system at 1780 K when thermochemical data[15] were used. The interface thickness 2 was taken as 6nm in the Fe-Mn-C alloy system at 1780 K and 60nm in the Fe-Cr-Ni alloy system, respectively. grid sizes of 1nm and 10nm for the above alloy systems were used so that the interfacial region ranged over six grid spacings. The total grid number was fixed at 200. The parameters of the phase field equation, and w were obtained from Eqs. (7) and (8). VD was assmumed to be 1 m/s. The diagonal terms of the solute diffusivity matrix were assumed to be the same in each phase and each component, which was assumed to be 3 10 9 . The off-diagonal terms were assumed to be zero for convenience, although the diffusivity matrix of each component in each phase could be obtained in a general way. The phase field mobility was obtained from Eq. (16). Figure 1 shows the evolution of the Cr and Ni concentration profiles at different times in the Fe-Cr-Ni alloy system, respectively. At 1 10 2 sec, the system reached equilibrium and at this time, the concentrations of Cr and Ni in solid phase were 8.829at% and 9.936at%, respectively and those in liquid phase were 10.031at% and 10.38at%,rescpectively. The equilibrium concentrations in our model showed good agreements with the equilibrium concentrations from thermodynamic data[15]. The equilibrium volume fraction of austenite and liquid phases are 0.8564 and 0.1436, respectively, which are almost identical to the values from thermodynamic data[15]. Fig. 1 The evolution of the concentration profile of Cr and Ni. The concentration profiles are shown at t= 0, 3.75 10 4 , 7.50 10 4 , 1.25 10 3 , 1.625 10 3 , 2.375 10 4 , 1.0 10 2 sec.. Figure 2 shows the evolution of the concentration profile of Mn and C at different 287 times for L/-ferrite solidification in the Fe-Mn-C alloy system at 1780K, respectively. At 5.88 10 5 sec, the system reached equilibrium and the Mn and C concentrations in the solid phase were 0.933at% and 0.263at%, respectively and in the liquid phase 1.263at% and 1.444at%, respectively. The equilibrium volume fractions of -ferrite and liquid were 0.797 and 0.203, respectively. The concentrations of Mn and C and the volume fraction of solid and liquid phase were in good agreements with the values from the thermodynamic data[15]. Fig. 2 The evolution of the concentration profile of Mn and C. The concentration profiles are shown at t= 0, 2.96 10 6 , 5.92 10 6 , 1.18 10 5 , 1.77 10 5 , 2.35 10 5 , 5.88 10 5 sec.. Fig. 3 Variations of interface concentrations, calculated as a function of distance * between the interface and a solute sink in a liquid, which engulfs all the solute influx from its neighbor. The solid line represents the calculated results from the present model and the filled circles are the results from the analytic solution (16) for the classical sharp interface model. Second, it will be shown that our model can realize the kinetics of solidification exactly. In order to study the kinetics of solidification, an 1D isothermal system reported by Kim, et al.[6,7] is adopted for steady state simulations. In addition, the alloy system for steady state solidification is adopted as a dilute solution so that the off-diagonal diffusivities of the solutes could be neglected in the dilute solutions. In the classical 288 sharp interface model with diffusion in liquid only, the exact solution[10] is as follows. (1 k ie )(e Vx / Dii e V L i c ( x) c c L i i 1 (1 k ie )(1 e V * * / DiiL / DiiL ) . (16) ) If V and * are given, the concentration profile of liquid phase can be obtained from Eq. (16) and the concentration at the interface can be determined at x 0 . The alloy system for the numerical calculations was adopted as Fe-1.0at%Mn-0.5at%C as used above. Figure 3 shows variations in the steady state Mn and C concentration at the interface, calculated as a function of the distance * between interface and solute sink. The system temperature was 1780K which is between the solidus and liquidus temperatures. The solid line is the calculated results from the present model and the solid circles are the results from the analytic solution (16) for the classical sharp interface model. The two results are in excellent agreement with each other. The error in the interface concentrations was within 1%. 3.2 Dendritic growth of a ternary alloy So far, it was shown that our model showed exactly the thermodynamic equilibrium and realized the kinetics of solidification. We have developed a 2-D calculation program based on the above verified 1-D model and applied it to the analysis of isothermal dendritic growth. In order to include the effects of anisotropy in our model, we follow the ideas reported by Kobayashi[1], Warren and Boettinger[5]. The detailed equations for 2-D dendritic growth can be found in Ref.[10]. The dendrite shapes of Fe-0.5mol%C binary alloy and Fe-0.5mol%C-0.005mol%Mn ternary alloy are shown in Fig. 4. The growth rate of the dendrite for the binary alloy is larger than that for the ternary alloy, which comes from the decrease of equilibrium liquidus and solidus lines due to the addition of the third element. In the ternary alloy, the secondary arms are more well-developed and complex in comparison with those of the binary alloy. The dendrite shape is significantly affected by a small amount of Mn. Because the diffusivity of Mn is much smaller than carbon, it is easy to be enriched in front of the interface and reduce the interface stability. The calculated pattern for the dendritic growth of binary and ternary alloys shows many features consistent with observations of dendritic growth of binary alloy presented by Warren and Boettinger[5]. First the spine of the primary stalk has a low concentration, with the regions of the dendrite between the secondary arms having the highest. Second, we note the tendency of the sidebranches to begin their growth in a direction not perpendicular to the primary stalk, and to later develop a growth axis perpendicular to the primary stalk. Behind the dendrite tip, the root of the side arm is 289 significantly narrower than the further out secondary arm. This feature is commonly observed in real dendrites. It is interesting to note the existence of spines of low concentration also on the secondary branches, which are as low as the spines in the primary stalks. Fig. 4 Plots of the C concentration profiles for the Fe-C and Fe-Mn-C alloys. The calculation was done on a 5001000 grid, and then reflected about y axis. The colors range from blue (lowest concentration) to green (central concentration) to blue (highest concentration). All the colors figures employ this color scheme. 4.CONCLUSION A phase field model is presented for the solidification of a multicomponent alloy system with a low interface velocity and a thin interface conditions. The model could be applicable to any alloy systems with both substitutional and interstitial alloy elements unlike the existing phase field model for the solidification of binary alloy systems. Because in multicomponent alloy systems, the interface kinetics coefficient could not be defined and the second response function does not have a simple form as in dilute binary alloy systems[14], the growth velocity from the chemical rate theory was used in order to determine the phase field mobility at the finite interface limit condition. This model was applied to simulate isothermal growth of large scale dendritc microstructures in a highly supersaturated liquid, and to predict the pattern of the solute distribution within the solid and liquid. The research presented in this paper is the first attempt to model the dendritic growth of multicomponent alloys. ACKNOWLEDGEMENTS The authors appreciate Prof. Kyu-Hwan Oh in Seoul National University for his help in thermodynamic evaluation and also Prof. Seong-Gyoon Kim in Kunsan National 290 University for his helpful discussions. Authors are grateful to the financial support of Brain Korea 21 program supported by Ministry of Education, Korea. Referencec 1. Kobayashi,R.: ‘Modeling and Numerical Simulations of Dendritic CrystalGrowth’ Physica D, 1993, 63(3-4), 410-423. 2. Karma,A., and Rappel,W.-J.: ‘Phase field method for computationally efficient modeling of solidification with arbitrary interface kinetics’ Phys. Rev. E, 1996, 53(4), R3017-R3020. 3. Karma,A., and Rappel,W.-J., ‘Quantitative phase-field modeling of dendritic growth in two and three dimensions’ Phys. Rev. E, 1998, 57(4), 4323-49. 4. Wheeler A. A., Boettinger W.J. and McFadden G.B: ‘Phase-field model for isothermal phase transitions in binary alloys’ Phys. Rev. A, 1992 45(10) 742439. 5. Warren,J.A. and Boettinger,W.J.: ‘Prediction of dendritic growth and microsegregation patterns in a binary alloy using the phase field method’ Acta metall. mater., 1995, 43(2), 689-703. 6. Kim S.G., Kim W.T., Suzuki T: ‘Interfacial compositions of solid and liquid in a phase-field model with finite interface thickness for isothermal solidification in binary alloys’ Phys. Rev. E, 1998 58(3) 3316-23 7. Kim,S.G., Kim,W.T. and Suzuki,T: ‘Phase-field model for binary alloys’, Phys. Rev. E, 1999, 60(6), 7186-97 8. Tiaden,J.,Nestler,B.,Diepers,H.J. and Steinbach,I.: ‘The multiphase-field model with an integrated concept for modelling solute diffusion’ Physica D, 1998, 115(1-2), 73-86. 9. Cha,P.-R.,Yeon,D.-H., and Yoon,J.-K.: ‘A phase field model for isothermal solidification of multicomponent alloys’ Phys. Rev. E, submitted. 10. Cha,P.-R.,Yeon,D.-H., and Yoon,J.-K.: ‘Prediction of dendritic growth in a ternary alloy using phase field model for isothermal solidification of multicomponent alloys’ Acta Mater., submitted. 11. Cahn,J.W. and Hilliard,J.E.: ‘Free energy of a nonuniform system. I. Interfacial free energy’ J. Chem. Phys., 1958, 28(2), 258-267. 12. Allen,S.M. and Cahn,J.W.: ‘A microscopic theory for antiphase boundary motion and its application to antiphase domain coarsening’ Acta metall., 1979, 27, 1085. 13. Hohenberg,P.C. and Halperin,B.I.: ‘Theory of dynamic critical phenomena’ Rev. Mod. Phys., 1977, 49(3), 435-479. 14. Ludwig,A.: ‘The interface response-functions in multi-componental alloy solidification’ Physica D, 1998, 124(1-3), 271-84. 291 15. Thermodynamic data for alloy systems in this study were obtained from thermodynamic software THERMO-CALC. 292