Limiting Reactant Practice: Stoichiometry Worksheet
advertisement

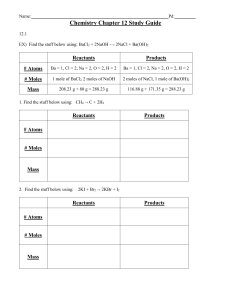
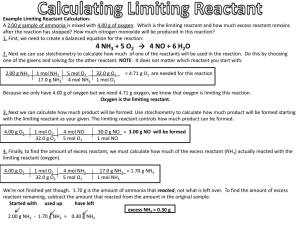
Chapter 12 Practice #3 Name ____________________________________ Limiting Reactant - The reactant in a chemical reaction that limits the amount of product that can be formed. The reaction will stop when all of the limiting reactant is consumed. Excess Reactant - The reactant in a chemical reaction that remains when a reaction stops when the limiting reactant is completely consumed. The excess reactant remains because there is nothing with which it can react. No matter how many tires there are, if there are only 8 car bodies, then only 8 cars can be made. Likewise with chemistry, if there is only a certain amount of one reactant available for a reaction, the reaction must stop when that reactant is consumed whether or not the other reactant has been used up. Example Limiting Reactant Calculation: A 2.00 g sample of ammonia is mixed with 4.00 g of oxygen. Which is the limiting reactant and how much excess reactant remains after the reaction has stopped? First, we need to create a balanced equation for the reaction: 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) Next we can use stoichiometry to calculate how much product is produced by each reactant. NOTE: It does not matter which product is chosen, but the same product must be used for both reactants so that the amounts can be compared. The reactant that produces the lesser amount of product: in this case the oxygen. Next, to find the amount of excess reactant, we must calculate how much of the non-limiting reactant (oxygen) actually did react with the limiting reactant (ammonia). We're not finished yet though. 1.70 g is the amount of ammonia that reacted, not what is left over. To find the amount of excess reactant remaining, subtract the amount that reacted from the amount in the original sample. Follow this example as you do the problems on the other side of this worksheet. 1. When copper is heated with an excess of sulfur, Cu2S is formed. How many g of Cu2S could be produced if 100.0 g of copper is heated with 50.0 g of sulfur? (Answer: 125 g) 2 Cu + S → Cu2S 2. A mixture containing 100 g of H2 and 100 g of O2 is ignited so that water is formed according to the reaction 2H2 + O2 → 2H2O. How much water is formed? (Answer 113 g H2O) How much excess remains? (Answer 87.4 g excess) 3. Calculate the mass of CCl4 which can be produced by the reaction of 10.0 g of carbon with 100.0 g of chlorine.( Answer: 108 g CCl4 ) Determine the mass of excess reagent left unreacted. (1.54 g left-over) C + 2Cl2 → CCl4 4. For the reaction 4Fe+ 3O2 → 2 Fe2O3, 4.80 g of oxygen is used to burin 0.150 mol of iron. What mass of Fe2O3 will be produced? (Answer 12.0 g Fe2O3 produced ) What mass of Fe will be left over at the end of the reaction? What mass of O2 will be left over at the end of the reaction? 5. For the balanced equation shown below, if 40.1 grams of C2H3O2Br were reacted with 8.01 grams of O2, how many grams of H2O would be produced? 2C2H3O2Br+O2 → 4CO+2H2O+2HBr 6. For the balanced equation shown below, if 84.4 grams of C2H3OF were reacted with 126 grams of O2, how many grams of F2 would be produced? 4C2H3OF+9O2 → 8CO2+6H2O+2F2 7. For the balanced equation shown below, if 58.6 grams of Al were reacted with 540 grams of H2SO4, how many grams of H2 would be produced? 2Al+3H2SO4 → Al2(SO4)3+3H2 8. For the balanced equation shown below, if 40.4 grams of C3H9N were reacted with 96.4 grams of O2, how many grams of NO2 would be produced? 4C3H9N+25O2 → 12CO2+18H2O+4NO2 Given the following balanced equation A. What mass of mercury is necessary to produce 23.7 g of mercury (I) oxide? B. What volume of oxygen gas will be required to produce the same amount of mercury (I) oxide at STP? 7. If 2.87 g of aluminum are reacted with excess copper (II) sulfate and 9.2 g of copper are produced what is the percent yield 2Al 3CuSO 4 Al 2 (SO 4 ) 3Cu 8. If 24.2 g of hydrogen react with excess oxygen and 3.45 g of water are produced what is the percent yield? 2H2 + O2 → 2H2O 9. When 3.8 g of aluminum are reacted with excess chlorine in the lab, you find you have made 7.8 g of aluminum chloride. What is the percent yield of this reaction? 2Al + 3Cl2→ 2AlCl3 10. If 2.35 moles of H2 gas react with 5.33 mol of N2 gas to make ammonia gas (NH3): N2 + 3H2 2 NH3 A. If you run out of H2 how many grams of NH3can you make? B. If you run out of N2 how many grams of NH3 can you make? C. In the mixture above, how many grams of NH3 can you make? D. What is the limiting reagent? E. How many moles of excess reagent will remain? 11. How many grams of NH3 can be produced if 28.0 g of N2 is reacted with 25.0 g of H2? N2 + 3H2 2 NH3 B. How much of the excess reagent were used? C. How much of the excess reagent is left over? 12. What mass of silver phosphate can be produced if 250 grams of silver nitrate and 250 g of sodium phosphate are mixed? 3 AgNO3 + Na3PO4 Ag3PO4 + 3NaNO3 13. How many grams of silver iodide can be produced from 52.38 g of iodine and unlimited silver? 14. If 46.2 g of sulfur trioxide decompose, how many liters of oxygen will be produced at STP? 15. A. How many grams of NH3 can be produced if 38.0 g of N2 is reacted with 11.2 g of H2 ? N2 + 3H2 2 NH3 B. In the reaction above, how much of the excess reagent is left over? 16. A chemist reacts a 50.6 g sample of Mg(OH)2 with 45.0 g of HCl according to the reaction: Mg(OH)2 + 2 HCl MgCl2 + 2 H2O The chemist actually obtains 55.4 g of MgCl2. What is the percent yield?