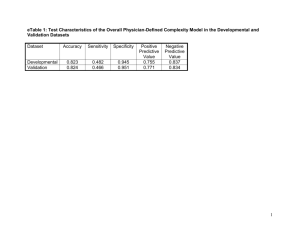
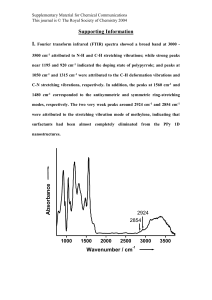
Supplementary Information (doc 2819K)
advertisement

Supporting Information Polypyrrole Functionalized PtPd Electrocatalysts with High Performance via the Formation of PtPd/PPy/PtPd Three-Layered Nanotube Arrays for Electrooxidation of Small Organic Molecules Han Xu,† Liang-Xin Ding,† Ye-Xiang Tong, and Gao-Ren Li* MOE Laboratory of Bioinorganic and Synthetic Chemistry, KLGHEI of Environment and Energy Chemistry, School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou 510275, China † These authors contributed equally to this work 5 nm Figure S1. HRTEM image of PPy layer in the marked green area in Figure 3c. 1 Figure S2. SEM image of PtPd nanotube arrays (NTAs). 2 PtPd/PPy/PtPd TNTAs PtPd/PPy/PtPd films (a) 1.0 Specific Current/mA cm-2 Specific Current/mA cm-2 2.0 1.2 (b) PtPd/PPy/PtPd TNTAs PtPd/PPy/PtPd films 1.5 0.8 0.6 0.4 @0.55V(vsSCE) 1.0 0.2 0.0 0.5 -0.2 -0.4 -0.6 0.0 -0.2 0.0 0.2 0.4 0.6 0.8 1.0 500 Potential/ V 1000 1500 2000 2500 3000 Time/sec (c) 0.92 Specific Current/ mA cm-2 0.69 0.46 PtPd/PPy/PtPd TNTAs 0.23 (d) 0.33 0.22 0.11 PtPd/PPy/PtPd films 0.00 0 100 200 300 400 500 Cycle Number Figure S3. (a) CVs of PtPd/PPy/PtPd TNTAs and PtPd/PPy/PtPd films in solution of 0.5 M CH3OH+0.5 M H2SO4 at 100 mV/s; (b) Chronoamperometry curves of PtPd/PPy/PtPd TNTAs and PtPd/PPy/PtPd films in solution of 0.5 M CH3OH + 0.5 M H2SO4 at 100 mV/s; (c) The change of peak current density with increasing cycle number for PtPd/PPy/PtPd TNTAs and PtPd/PPy/PtPd films. 3 The electrocatalytic activity of PtPd/PPy/PtPd TNTAs towards methanol oxidation along with PtPd/ PPy/PtPd films was studied in solution of 0.5 M CH3OH+0.5 M H2SO4, and the CVs at 100 mV/s are shown in Figure S3a, which shows the specific electroactivity of PtPd/PPy/PtPd TNTAs is ~2.7 times higher than that of PtPd/PPy/PtPd films. The chronoamperometry curves of PtPd/PP/PtPd TNTAs and PtPd/PPy/PtPd films were also measured in solution of 0.5 M CH3OH + 0.5 M H2SO4 as shown in Figure S3b. Compared with PtPd/PPy/PtPd films, it is obvious that the PtPd/ PPy/PtPd TNTAs exhibit a much higher current density over time and a much slower attenuation, indicating a much higher catalytic activity for methanol electrooxidation and a much higher tolerance to carbonaceous species generated during methanol electrooxidation. In addition, the PtPd/PPy/PtPd TNTAs also show higher durability than PtPd/PPy/PtPd films as shown in Figure S3c. 4 PtPd/PPy/PtPd TNTAs PtPd/PPy/PtPd films (a) -0.2 0.0 0.2 0.4 0.6 0.8 Specific Current/mA cm-2 Specific Current/mA cm-2 2.0 1.8 1.6 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 -0.2 -0.4 1.0 (b) 1.5 PtPd/PPy/PtPd TNTAs PtPd/PPy/PtPd films @0.20V(vs SCE) 1.0 0.5 0.0 500 1000 1500 2000 2500 3000 Time/sec Potential/ V (c) 1.62 Specific Current/ mA cm-2 1.44 1.26 PtPd/PPy/PtPd TNTAs 1.08 (d) 0.76 PtPd/PPy/PtPd films 0.57 0.38 0.19 0 50 100 150 200 250 Cycle Number Figure S4. (a) CVs of PtPd/PPy/PtPd TNTAs and PtPd/PPy/PtPd films in solution of 0.5 M HCOOH+0.5 M H2SO4 at 100 mV/s; (b) Chronoamperometry curves of PtPd/PPy/PtPd TNTAs and PtPd/PPy/PtPd films in solution of 0.5 M HCOOH+0.5 M H2SO4 at 100 mV/s; (c) The change of peak current density with increasing cycle number for PtPd/PPy/PtPd TNTAs and PtPd/PPy/PtPd films. 5 3 (a) 2.5 (b) 2.0 2 1 PtPd/PPy/PtPd TNTAs PtPd/PPy/PtPd films @0.65V(vsSCE) 1.5 0 1.0 -1 PtPd/PPy/PtPd TNTAs PtPd/PPy/PtPd films -2 -3 -4 Specific Current/mA cm-2 Specific Current/mA cm-2 4 0.5 0.0 -0.2 0.0 0.2 0.4 0.6 0.8 1.0 500 1000 1500 2000 2500 3000 Time/sec Potential/ V 2.66 (c) Specific Current/ mA cm-2 2.28 1.90 PtPd/PPy/PtPd TNTAs 1.52 0.85 (d) 0.68 0.51 PtPd/PPy/PtPd films 0.34 0 50 100 150 200 250 Cycle Number Figure S5. (a) CVs of PtPd/PPy/PtPd TNTAs and PtPd/PPy/PtPd films in solution of 0.5 M CH3CH2OH +0.5 M H2SO4 at 100 mV/s; (b) Chronoamperometry curves of PtPd/PPy/PtPd TNTAs and PtPd/PPy/PtPd films in solution of 0.5 M CH3CH2OH +0.5 M H2SO4 at 100 mV/s; (c) The change of peak current density with increasing cycle number for PtPd/PPy/PtPd TNTAs and PtPd/PPy/PtPd films. 6 2.0 (a) (b) -2 -2 1.0 1.5 0.6 Specific Current/mA cm Specific Current/ mA cm 0.8 0.4 PtPd/PPy two-layered nanotube arrays 1.0 0.2 0.0 -0.2 PtPd/PPy/PtPd TNTAs @0.55V(vsSCE) 0.5 PtPd/PPy/PtPd TNTAs -0.4 PtPd/PPy two-layered nanotube arrays -0.6 0.0 -0.2 0.0 0.2 0.4 0.6 0.8 500 1.0 1000 1500 2000 2500 3000 Time/sec Potential/ V (c) 0.92 -2 0.69 Specific Current/ mA cm 0.46 PtPd/PPy/PtPd TNTAs 0.23 (d) 0.54 0.36 PtPd/PPy two-layered nanotube arrays 0.18 0.00 0 100 200 300 400 500 Cycle Number Figure S6. (a) CVs of PtPd/PPy/PtPd TNTAs and PtPd/PPy two-layered nanotube arrays in solution of 0.5 M CH3OH+0.5 M H2SO4 at 100 mV/s; (b) Chronoamperometry curves of PtPd/PPy/PtPd TNTAs and PtPd/PPy two- layered nanotube arrays in solution of 0.5 M CH3OH+0.5 M H2SO4 at 100 mV/s; (c) The change of peak current density with increasing cycle number for PtPd/PPy/PtPd TNTAs and PtPd/PPy two-layered nanotube arrays. 7 PtPd/PPy/PtPd TNTAs (a) 1.8 PtPd/PPy two-layered nanotube arrays Specific Current/mA cm 1.6 1.4 1.2 1.0 Specific Current/mA cm-2 -2 2.0 (b) 1.5 PtPd/PPy/PtPd TNTAs PtPd/PPy two-layered nanotube arrays @0.20V(vs SCE) 1.0 0.8 0.6 0.4 0.5 0.2 0.0 -0.2 -0.4 -0.2 0.0 0.2 0.4 0.6 0.8 0.0 1.0 Potential/ V 500 1000 1500 2000 2500 3000 Time/sec (c) 1.62 -2 1.44 1.26 Specific Current/ mA cm PtPd/PPy/PtPd TNTAs 1.08 1.25 (d) PtPd/PPy two-layered nanotube arrays 1.00 0.75 0.50 0 50 100 150 200 250 Cycle Number Figure S7. (a) CVs of PtPd/PPy/PtPd TNTAs and PtPd/PPy two-layered nanotube arrays in solution of 0.5 M HCOOH+0.5 M H2SO4 at 100 mV/s; (b) Chronoamperometry curves of PtPd/PPy/PtPd TNTAs and PtPd/PPy two-layered nanotube arrays in solution of 0.5 M HCOOH+0.5 M H2SO4 at 100 mV/s; (c) The change of peak current density with increasing cycle number for PtPd/PPy/PtPd TNTAs and PtPd/PPy two-layered nanotube arrays. 8 4 (a) -2 -2 3 2.5 (b) 2.0 1 Specific Current/mA cm Specific Current/mA cm 2 0 PtPd/PPy two-layered nanotube arrays 1.5 -1 -2 PtPd/PPy/PtPd TNTAs @0.65V(vsSCE) 1.0 -3 -4 PtPd/PPy/PtPd TNTAs -5 PtPd/PPy two-layered nanotube arrays 0.5 -6 0.0 -0.2 0.0 0.2 0.4 0.6 0.8 1.0 500 1000 1500 2000 2500 3000 Time/sec Potential/ V 2.66 (c) -2 2.28 Specific Current/ mA cm 1.90 PtPd/PPy/PtPd TNTAs 1.52 (d) 1.740 1.653 1.566 PtPd/PPy two-layered nanotube arrays 1.479 0 50 100 150 200 250 Cycle Number Figure S8. (a) CVs of PtPd/PPy/PtPd TNTAs and PtPd/PPy two-layered nanotube arrays in solution of 0.5 M CH3CH2OH +0.5 M H2SO4 at 100 mV/s; (b) Chronoamperometry curves of PtPd/PPy/PtPd TNTAs and PtPd/PPy two-layered nanotube arrays in solution of 0.5 M CH3CH2OH +0.5 M H2SO4 at 100 mV/s; (c) The change of peak current density with increasing cycle number for PtPd/PPy/PtPd TNTAs and PtPd/PPy two-layered nanotube arrays. 9