determining equivalent weight of an organic acid or base
advertisement

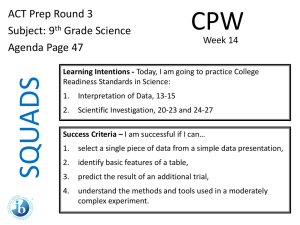
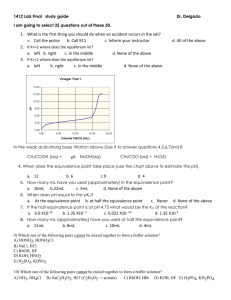
DETERMINING EQUIVALENT WEIGHT OF AN ORGANIC ACID OR BASE Acids and bases with ionization constants of greater than about 10-7 to 10-8 (pK values < 7-8), and which are soluble in water (i.e., Sa, Sb, and S2 groups) can be titrated with good accuracy in aqueous media using standard solutions of strong acids (aq. HCl, etc.) or strong bases (aq. NaOH). Familiar examples include titrimetric analysis of acetic acid with aq. NaOH and titrimetric analysis of potassium hydrogen phthalate with aq. HCl. In the event that an organic acid is sufficiently strong (pKa < 8) but is insoluble in water it may dissolve as it reacts with aq. NaOH titrant (forming a water soluble salt) and give a good endpoint. it may be able to be titrated using aq. NaOH titrant after dissolving the sample in methanol. (Check the CRC handbook for the solubility of the compound if its identity is known. If the identity is not known, try dissolving it in methanol. Ethanol and acetone are other possible solvents). Use small volumes of nonaqueous solvents. In the event that an organic basic is sufficiently strong (pKb < 8) but is insoluble in water it may dissolve as it reacts with aq. HCl titrant (forming a water soluble salt) and give a good endpoint. it may be able to be titrated using aq. HCl titrant after dissolving the sample in methanol. (Check the CRC handbook for the solubility of the compound if its identity is known. If the identity is not known, try dissolving it in methanol. Ethanol and acetone are other possible solvents). Use small volumes of nonaqueous solvents. Acids and bases with ionization constants less than about 10-7 to 10-8 (pK values > 7-8) cannot be accurately titrated in aqueous media because water interferes in several ways: the large quantity of HOH solvent reverses the reaction (Le Chatalier) HOH is amphiprotic - both weakly acidic and weakly basic (pKa = pKb = 15.74) and tends to reverse titrations of weak acids or weak bases near the end point. For acids or bases with pK values > 7-8, any attempts at titration in aqueous media will yield indefinite, fading endpoints with poor reproducibility. An excess of titrant will be required to bring the reaction to completion - but this is unsuitable for determining equivalent weight. In such cases, a nonaqueous solvent (solvent other than water) with a higher pK value is required. Replacing water with methanol may help somewhat by reducing the amount of HOH at the end point however methanol has about the same acidity (pKa = 15.2) and basicity (pKb = 16.0) as water and will also interfere with the titration of very weak acids or bases. Other nonaqueous solvents include: ethanol (pKa = 16, pKb = 17.6), t-butanol (pKa = 16, pKb = 18), acetic acid (pKa = 4.7, pKb = 20), acetone (pKa = 19.3, pKb = 21.2), and acetonitrile (pKa = >20, pKb = 24) Another immediately obvious complication of using a nonaqueous solvent is that our traditional inorganic titrants (NaOH and HCl) may not be soluble or readily prepared as solutions in the organic, nonaqueous solvent. Details of nonaqueous titrations are beyond the scope of this course but can be found in many analytical chemistry texts. We can state, as a rule of thumb, that in order to conduct a stoichiometric titration of an acid and base in aqueous media, [ pKa (of the acid) + pKb (of the base) must be 6 ] or [ pKeq must be -8 ].