1 Zielsetzung - Royal Society of Chemistry
advertisement
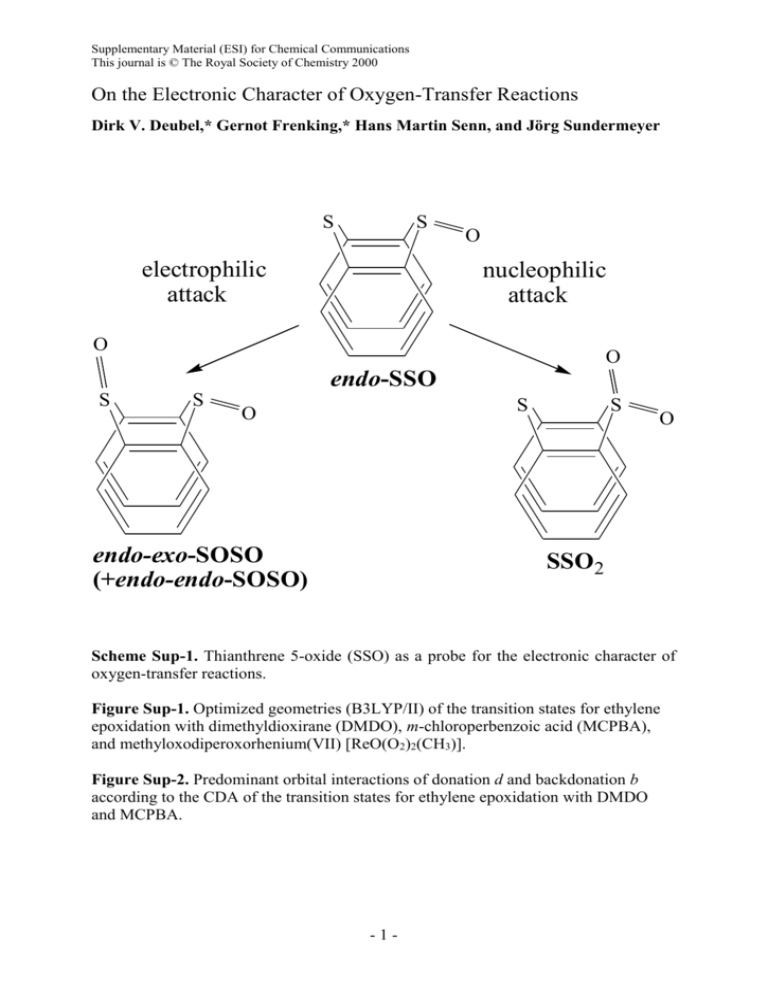
Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 On the Electronic Character of Oxygen-Transfer Reactions Dirk V. Deubel,* Gernot Frenking,* Hans Martin Senn, and Jörg Sundermeyer S S electrophilic attack O nucleophilic attack O O endo-SSO S S S O endo-exo-SOSO (+endo-endo-SOSO) S O SSO2 Scheme Sup-1. Thianthrene 5-oxide (SSO) as a probe for the electronic character of oxygen-transfer reactions. Figure Sup-1. Optimized geometries (B3LYP/II) of the transition states for ethylene epoxidation with dimethyldioxirane (DMDO), m-chloroperbenzoic acid (MCPBA), and methyloxodiperoxorhenium(VII) [ReO(O2)2(CH3)]. Figure Sup-2. Predominant orbital interactions of donation d and backdonation b according to the CDA of the transition states for ethylene epoxidation with DMDO and MCPBA. -1- Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 Standard density functional (DFT) methods for oxidations: (1) For geometry optimizations, Becke’s three-parameter hybrid functional (B3)3 together with the correlation functional of Lee, Yang, and Parr (LYP)4 were employed as implemented in Gaussian 98.5 Our standard basis set II6 was used, which consists of relativistic small-core ECPs7 and valence basis set splittings (441/2111/21) for Re and (441/2111/31) for Mo, while the 6-31G(d)8 allelectron basis set was employed for the other atoms. All stationary points were characterized by the calculation of vibrational frequencies. The zero-point energy (ZPE) corrections are unscaled. Improved total energies were calculated at the B3LYP level using the same ECP and valence basis set at Re and Mo, but totally uncontracted and augmented with one set of f-type polarization functions ( = 0.869 for Re and = 1.043 for Mo),9 together with the 6-31+G(d) basis set at the other atoms.10 This basis-set combination is denoted III+.2 (2) Deubel, D. V.; Frenking, G. J. Am. Chem. Soc. 1999, 121, 2021. (3) Becke, A. D. J. Chem. Phys. 1993, 98, 5648. (4) Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785. (5) Gaussian 98 (Revision A.3): Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, J. A.; Stratmann, R. E.; Burant, J. C.; Dapprich. S.; Milliam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomberts, R.; Martin, R. L.; Fox, D. J.; Keith, T. A.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M. W.; Johnson, B. G.; Chen, W.; Wong, M. W.; Andres, J. L.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian Inc., Pittsburgh, PA, 1998 (6) Frenking, G.; Antes, I.; Böhme, M.; Dapprich, S.; Ehlers, A. W.; Jonas, V.; Neuhaus, A.; Otto, M.; Stegmann, R.; Veldkamp, A.; Vyboishchikov, S. F. in Reviews in Computational Chemistry; Lipkowitz, K. B.; Boyd, D. B. Eds.; VCH: New York, 1996, Vol. 8, p. 63. (7) Hay, P. J.; Wadt, W. R. J. Chem. Phys. 1985, 82, 299. (8) (a) Binkley, J. S.; Pople, J. A.; Hehre, W. J. J. Am. Chem. Soc. 1980, 102, 939. (b) Hehre, W. J.; Ditchfield, R.; Pople, J. A. J. Chem. Phys. 1972, 56, 2257. -2- Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2000 (9) Ehlers, A. W.; Böhme, M.; Dapprich, S.; Gobbi, A.; Höllwarth, A.; Jonas, V.; Köhler, K. F.; Stegmann, R.; Veldkamp, A.; Frenking, G. Chem. Phys. Lett. 1993, 208, 111. (10) Clark, T.; Chandrasekhar, J.; Spitznagel, G. W.; Schleyer, P. v. R. J. Comput. Chem. 1983, 4, 294. -3-