Protocol
advertisement

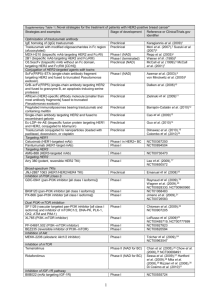
Open Protocols Stamford Hospital/Medical Oncology Hematology Research Primary Breast Disease Stage Adjuvant Breast Adjuvant Digestive/GI Colon Adjuvant Digestive/GI Colorectal Protocol NCIC MA.32 Dr. Angevine Mary Miller, LPN NSABP B-49 Dr. Weinstein Mary Miller, LPN NSABP P-5 Dr. Del Prete Ed Hatton, RN Genentech ML25710 Dr. Del Prete Ed Hatton, RN Title A Phase III Randomized Trial of Metformin versus Placebo on Recurrence and Survival in Early Stage Breast Cancer A Phase III Clinical Trial Comparing the Combination of Docetaxel Plus Cyclophosphamide to Anthracycline-Based Chemotherapy Regimens for Women with Node-Positive or High-Risk Node-Negative, HER2-Negative Breast Cancer Statin Polyp Prevention Trial in Patients with Resected Colon Cancer A Randomized Phase II Study of Bevacizumab/mFOLFOX6 vs. Bevacizumab/FOLFIRI with Biomarker Stratification in Patients with Previously Untreated Metastatic Colorectal Cancer (MAVERICC) Digestive/GI Colorectal Gilead GS-US-205-0203 Dr. Del Prete Ed Hatton, RN A Phase 2 Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of GS-6624 Combined with FOLFIRI as Second Line Treatment for Metastatic KRAS Mutant Colorectal Adenocarcinoma that has Progressed Following a First Line Oxaliplatin- and Fluoropyrimidine-Containing Regimen Digestive/GI Colorectal Metastatic NCCTG N0949 Dr. Del Prete Ed Hatton, RN Randomized Phase III Trial of mFOLFOX7 or XELOX Plus Bevacizumab Versus 5-Fluorouracil/Leucovorin or Capecitabine Plus Bevacizumab as First-line Treatment in Elderly Patients with Metastatic Colorectal Cancer Digestive/GI Pancreatic Metastatic Gilead GS-US-324-0101 Dr. Del Prete Ed Hatton, RN A Phase 2 Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of GS-6624 Combined with Gemcitabine as First Line Treatment for Metastatic Pancreatic Adenocarcinoma Digestive/GI Pancreatic Recurrent or Metastatic INCYTE INCB 18424-262 Dr. Del Prete Ed Hatton, RN A Randomized Phase 2 Study of Ruxolitinib Efficacy and Safety in Combination with CApecitabine for Subjects with recurrent or Treatment Refractory Metastatic Pancreatic Cancer (The RECAP trial) Digestive/GI Pancreatic Adjuvant Stage I or II Resected R0 or R1 NewLink NLG-0405 Dr. Del Prete Ed Hatton, RN Metastatic CALGB 90601 Dr. Cohen Ed Hatton, RN Genitourinary/GU Bladder A Phase III Study of Chemotherapy and Chemoradiotherapy With or Without HyperAcute®-Pancreas (algenpantucel-L) Immunotherapy is Subjects with Surgically Resected Pancreatic Cancer A Randomized Doubled-Blinded Phase III Study Comparing Gemcitabine, Cisplatin, and Bevacizumab to Gemcitabine, Cisplatin, and Placebo in Patients with Advanced Transitional Cell Carcinoma D:\533569943.doc 11JAN12, 2FEB12, 20Feb12; 8MAR12, 22MAY12, 24MAY12, 4JUN12, 7JUN12 Copy to: C: Builes, C. Di Bona, D. Derdelinghen, L. Manfredo, , K. Radziewicz, M. Ronk, D. Sardanovic, E. Johnson Page 1 of 3 Open Protocols Stamford Hospital/Medical Oncology Hematology Research Primary Disease Stage Protocol Genitourinary/GU Prostate Dendron P10-3 Dr. Cohen Mary Miller, LPN Genitourinary/GU Prostate Sanofi-Aventis EFC11784 Dr. Cohen Ed Hatton, RN Genitournary/GU Prostate Sanofi-Aventis EFC11785 Dr. Cohen Ed Hatton, RN Genitourinary/GU Renal Metastatic GYN - Ovarian, Primary Peritoneal, or Fallopian Tube Cancers GYN Ovarian CALGB 90802 Dr. Del Prete Sue Murdock, RN Amgen 20101129 Dr. Del Prete Sue Murdock, RN II-IV or recurrent stage I GOG-0241 Dr.Del Prete Sue Murdock, RN GYN Ovarian Merrimack MM-121-04-02-08 Dr. Del Prete Sue Murdock, RN GYN Uterus GOG-0261 Dr.Del Prete Sue Murdock, RN I-IV Title A Registry of Sipuleucel-T® Therapy in Men with Advanced Prostate Cancer (PROCEED) Randomized, Open Label, Multi-Center Study comparing Cabazitaxel at 25 mg/m2 and at 20 mg/m2 in combination with Prednisone Every 3 Weeks to Docetaxel in Combination with Prednisone in Patients with Metastatic Castration Resistant Prostate Cancer not Pretreated with Chemotherapy (Study Name: FIRSTANA) Randomized, Open-Label Multi-Center Study comparing Cabazitaxel at 20 mg/m2 and at 25 mg/m2 Every 3 Weeks in Combination with Prednisone for the Treatment of Metastatic Castration Resistant Prostate Cancer Previously Treated with a Docetaxel-Containing Regimen (Study Name: PROSELICA) Randomized Phase III Trial Comparing Everolimus Plus Placebo Versus Everolimus Plus Bevacizumab For Advanced Renal Cell Carcinoma Progressing After Treatment With Tyrosine Kinase Inhibitors A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multi-Center Study of AMG 386 with Paclitaxel and Carboplatin as First-line Treatment of Subjects with Figo Stage III-IV Epithelial Ovarian, Primary Peritoneal or Fallopian Tube Cancers (TRINOVA-3) A GCIG Intergroup Multicenter Phase III Trial of Open Label Carboplatin and Paclitaxel +/- NCI-Supplied Agent: Bevacizumab (NSC #704865, IND #113912) Compared with Oxaliplatin and Capecitabine +/- Bevacizumab as First Line Chemotherapy in Patients with Mucinous Epithelial Ovarian or Fallopian Tube Cancer (MEOC) A Phase II Randomized Open Label Study of MM-121 in Combination with Paclitaxel Versus Paclitaxel Alone in Patients with Platinum Resistant/Refractory Advanced Ovarian Cancers A Randomized Phase III Trial of Paclitaxel Plus Carboplatin versus Ifosfamide Plus Paclitaxel in Chemotherapy-Naïve Patients with Newly Diagnosed Stage I-IV Persistent or Recurrent Carcinosarcoma (Mixed Mesodermal Tumors) of the Uterus Connect™ CLL: The Chronic Lymphocytic Leukemia Disease Registry Celgene Connect CLL Dr. Bar Sue Murdock, RN Hematologic Malignancy Lymphoma Diffuse Large Cell Therapeutics PIX306 A Randomized Multicenter Study Comparing Pixantrone + Rituximab with D:\533569943.doc 11JAN12, 2FEB12, 20Feb12; 8MAR12, 22MAY12, 24MAY12, 4JUN12, 7JUN12 Copy to: C: Builes, C. Di Bona, D. Derdelinghen, L. Manfredo, , K. Radziewicz, M. Ronk, D. Sardanovic, E. Johnson Page 2 of 3 Open Protocols Stamford Hospital/Medical Oncology Hematology Research Primary Disease Stage Protocol B-Cell Dr. Angevine Sue Murdock, RN Gemcitabine + Rituximab in Patients with Aggressive B-Cell Non-Hodgkin’s Lymphoma Who Have Relapsed after Therapy with CHOP-R or an Equivalent Regimen and are Ineligible for Stem Cell Transplant MMRF-11-001 Dr. Bar Sue Murdock, RN A Prospective, Longitudinal, Observational Study in Newly Diagnosed Multiple Myeloma (MM) Patients to Assess the Relationship between Patient Outcomes, Treatment Regimens and Molecular Profiles (CoMMpass) Multiple Myeloma Pulmonary/Lung NSCLC Adjuvant IB-IIIA Genentech GO27912 Pulmonary/Lung NSCLC Dr. Del Prete Ed Hatton, RN Pulmonary/Lung Melanoma ECOG 1505 Dr. Del Prete Ed Hatton, RN Stage III/IV Millennium C14007 Dr. Del Prete Mary Miller, LPN E1609 Dr. Del Prete Ed Hatton, RN Title A Phase III Randomized Trial of Adjuvant Chemotherapy With or Without Bevacizumab for Patients with Completely Resected Stage IB (≥ 4cm) – IIA NonSmall Cell Lung Cancer (NSCLC) A Phase II, Double-Blind, Placebo-Controlled, Randomized Study Evaluating the Safety and Efficacy of Carboplatin/Paclitaxel and Carboplatin/Paclitaxel/Bevacizumab with and without GDC-0941 in Patients with Previously Untreated Advanced or Recurrent Non-Small Cell Lung Cancer A Phase I Dose Escalation Study of MLN8237, an Aurora A Kinase Inhibitor, in Adult Patients With Non-hematological Malignancies, Followed by a Phase 2 of MLN8237 in Lung, Breast, Head and Neck, or Gastroesophageal Malignancies A Phase III Randomized Study of Adjuvant Ipilimumab Anti-CTLA4 Therapy versus High-Dose Interferon α-2b for Resected High-Risk Melanoma D:\533569943.doc 11JAN12, 2FEB12, 20Feb12; 8MAR12, 22MAY12, 24MAY12, 4JUN12, 7JUN12 Copy to: C: Builes, C. Di Bona, D. Derdelinghen, L. Manfredo, , K. Radziewicz, M. Ronk, D. Sardanovic, E. Johnson Page 3 of 3