to open the MS Word version of the Quarterly Action Agenda
advertisement

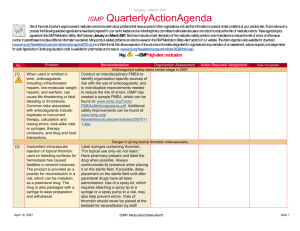
July - September 2014 ISMP QuarterlyActionAgenda One of the most important ways to prevent medication errors is to learn about problems that have occurred in other organizations and to use that information to prevent similar problems at your practice site. To promote such a process, the following selected items from the July—September 2014 issues of the ISMP Medication Safety Alert! have been prepared for an interdisciplinary committee to stimulate discussion and action to reduce the risk of medication errors. Each item includes a brief description of the medication safety problem, a few recommendations to reduce the risk of errors, and the issue number to locate additional information as desired. Look for our high-alert medication icon under the issue number if the agenda item involves one or more medications on the ISMP List of High-Alert Medications (www.ismp.org/Tools/highalertmedications.pdf). The Action Agenda is also available for download in a Microsoft Word format (www.ismp.org/Newsletters/acutecare/articles/ActionAgenda1404.doc) that allows expansion of the columns in the table designated for organizational documentation of an assessment, actions required, and assignments for each agenda item. Many product-related problems can also be viewed in the ISMP Medication Safety Alert! section of our website at: www.ismp.org. Continuing education credit is available for nurses at: www.ismp.org/Newsletters/acutecare/actionagendas.asp. Key: — ISMP high-alert medication Organization Assessment Error with lomustine caused by dispensing more than a single course of therapy No. Problem (14) A patient with brain cancer was prescribed a single 150 mg dose of lomustine, followed by reassessment in 6 weeks to evaluate the benefit of additional doses. A mail-order pharmacy dispensed a 3-cycle supply of the drug (3 doses). Expecting a single dose, the patient took all of the capsules totaling 450 mg. The overdose led to a slow and painful death 6 weeks later. Similar errors with dispensing this drug have been published previously with fatal results. (16, 20) Early in 2015, manufacturers will distribute administration sets with the new enteralonly connector, called ENFit, at the end that connects to the feed-ing tube. The new feeding tubes will not be available until later in 2015. Thus, a temporary transition adapter will be attached to the administration set. Once new enteral feeding tubes are in place, ENFit syringes will be required to flush or administer enteral liquid medications via a feeding tube. An oral or Luer syringe will not connect to the port. Recommendation Action Required/ Assignment Date Completed Order entry systems should not allow more than a single dose to be entered and should limit the quantity dispensed. Pharmacists should provide patient counseling, supply leaflets, and enhance labels (e.g., CAUTION: TAKE A SINGLE DOSE ONLY ONCE EVERY 6 WEEKS). Nurses should reinforce education prior to discharge, and insurers should pay only for a single dose at a time. URGENT! Health systems need to plan for changes in enteral connectors October 23, 2014 Convene an interdisciplinary team to assess the existing systems and processes that may need to change during and after transition to the new enteral connectors. The team should specifically focus on how the change will be communicated, how the enteral medications will be dispensed, how the connectors and sets will be stored, and how to transition to the new connectors. To stay updated, visit this website frequently: www.stayconnected2014.org/. ISMP MedicationSafetyAlert! QAA 1 July - September 2014 ISMP QuarterlyActionAgenda Organization Assessment Mix-ups between vaccine diluents and neuromuscular blockers No. Problem (19) In the past, vaccine diluents have been mixed up with neuromuscular blockers primarily due to look-alike vials and ampules, as happened recently in Syria, leading to the death of 15 children vaccinated against measles. Similar errors have happened around the world and in the US where several patients received doses of pancuronium instead of influenza vaccine because the similar vials were stored in the refrigerator next to each other. ISMP has called upon regulators and manufacturers to improve vaccine packaging, including the use of dualchamber containers and redesigned vials that allow larger labels. Until then, when pos-sible, stock prefilled vaccine syringes to decrease reconstitution in patient care units. Limit or eliminate the storage of neuromuscular blockers on patient care units and sequester remaining storage. Educate staff about vaccine and diluent errors. (16) Two children sustained leg lacerations while receiving EpiPen injections when they moved while the needle was still under the skin. The directions for use say that the EpiPen must be held in place for 10 seconds. The needle does not retract until the pen is removed. The needle can cut through the subcutaneous tissue and cause a laceration if the child moves during administration. Leg lacerations following EPIPEN (EPINEPHrine) autoinjector use Providers using EPINEPHrine autoinjectors for children should pay close attention to patient restraint prior to injection, as the child may move if the needle remains in the thigh for 10 seconds. If the needle is dislodged, reinsertion should never be attempted. If it was in place for at least 3 seconds, the EPINEPHrine was likely delivered. Repeat doses only if clinically necessary. (13, 16) Recommendation Action Required/ Assignment Date Completed Confusion between NAROPIN (ropivacaine) and OFIRMEV (acetaminophen injection) Glass vials of Naropin, intended for Consider adding auxiliary labels (“For epidural use, have been repeatedly nerve block and epidural use only”) to the confused with glass vials of Ofirmev, Naropin containers before dispensing the intended for IV use. Either product can be product from the pharmacy. Store the administered without further dilution. products in different drawers or, when Although, the labels are dissimilar, the possible, limit ADC storage to only one of drugs may be the only two available in the drugs. Use barcode scanning for similar glass vials in some patient care stocking and removal of the products from areas. The risk of error is highest if they ADCs. are stored near one another, such as in the same automated dispensing cabinet (ADC) drawer. October 23, 2014 ISMP MedicationSafetyAlert! QAA 2 July - September 2014 ISMP Organization Assessment Baxter DOBUTamine overwrap label change contributes to error Problem No. Recommendation (13) With the new labeling on Baxter’s DOBUTamine IV bags, the concentration appears on the overwrap but there’s no longer reverse print used for the 250 mg/250 mL bag to differentiate it from the 500 mg/250 mL bag. A mix-up happened when 500 mg/250 mL bags were accidentally used in place of the lower concentration to restock an automated dispensing cabinet (ADC). (13) To prepare an 850 mg dose of Alimta, a total of 34 mL were needed using one 500 mg vial and four 100 mg vials after being reconstituted to obtain a final concentration of 25 mg/mL. But pharmacy staff were able to obtain 34 mL of Alimta without using the last 100 mg vial of drug. When contacted, Lilly confirmed that the vials contain overfill. In this instance, one vial was wasted, but the patient could have received a larger dose than prescribed. (16, 17) QuarterlyActionAgenda Action Required/ Assignment Date Completed Standardize to a single concentration when possible and use barcode scanning when restocking or removing the product from ADCs. In hospitals where more than one concentration must remain, consider storing only one concentration in the ADC, and dispense the less common strength from the pharmacy. Add bold auxiliary labels to draw attention to the concentration differences. ALIMTA (PEMEtrexed) vials contain overfill Overfill is mentioned in the package insert, but the details aren’t specified. Be sure staff are aware of vial overages with this drug, especially when a small difference could be clinically important. New ISOVUE (iopamidol) imaging bulk package (IBP) for use with power injectors Isovue is now packaged in an imaging bulk The Isovue PBP and Isovue IBP products package (IBP) approved for use with must be used exactly as described in the multiple patients in the CT suite in package insert to ensure sterility. conjunction with an automated contrast injection system or syringe-based injection system. Use of the Isovue Multipack PBP (pharmacy bulk package), renamed Isovue PBP, is limited to the preparation of syringes in an ISO Class 5 environment in the pharmacy. Misleading KCENTRA (prothrombin complex concentrate [human]) label leads to dosage errors October 23, 2014 ISMP MedicationSafetyAlert! QAA 3 July - September 2014 ISMP QuarterlyActionAgenda Organization Assessment No. Problem Recommendation (15) When 4,500 units of Kcentra were ordered, pharmacy staff noted the “500 U” in the upper right corner of the carton and vial label and assumed each vial contained 500 units. Each vial actually contains a range (400-620 units) of factor IX, which is listed on the back panel or the carton and vial label. Nine vials were used to prepare an infusion labeled “4,500 units in 180 mL,” but the bag contained 5,026 units given the actual units in each vial. Use an auxiliary label or computer reminder to alert staff to verify the actual number of units in each vial listed on the back label panel on the carton and vial. We asked the company to modify the label so the range is better communicated. We also suggested that additional information is needed where “500 U” appears on the front label panel—perhaps specifying “500 Units Range” as a midpoint of dosages in each vial. (19) The Docefrez brand of DOCEtaxel is available as a lyophilized powder that reconstitutes to a concentration different than most commercially available DOCEtaxel products. Instead of a 10 mg/mL or 20 mg/mL concentration, reconstitution results in 24 mg/mL (80 mg vial) or 25 mg/mL (20 mg vial) concentrations. Overdoses have occurred, as the concentration is not clearly visible on the label. Action Required/ Assignment Date Completed Unfamiliar concentration of DOCEFREZ (DOCEtaxel) increases risk of error (15) List Docefrez along with the concentration after reconstitution in order entry systems. Have a process in place to review new products (particularly for replacement products on backorder or during drug shortages) so that staff are aware of the differences, and make adjustments to the IT system (including strengths and concentrations) as needed for safe use. “Once-Daily” designation on label of long-acting ASTAGRAF (tacrolimus) leads to error A renal transplant patient who had been Properly label containers dispensed to taking oral tacrolimus 3 mg every 12 hours patients and provide education to them was converted to a once-daily product, about how to take the medicine. ISMP Astagraf, and instructed to take 6 mg. reported this risk to FDA for consideration Despite application of a pharmacy label, of label modifications. the manufacturer’s label was visible, which prominently displays “ONCE-DAILY” below the drug name and 1 mg strength. The patient took just 1 mg once daily. October 23, 2014 ISMP MedicationSafetyAlert! QAA 4 July - September 2014 ISMP No. Problem (17) Dantrolene, used to treat malignant hyperthermia (MH), is often available in kits containing 20 mg vials of the drug and a liter bag of sterile water to dilute each vial with 60 mL. Preparation during an emergency takes time because multiple 20 mg vials are often needed. Accidental IV administration of the liter bag of sterile water as also occurred with catastrophic results. (17) QuarterlyActionAgenda Organization Assessment New dantrolene (RYANODEX) product can improve safety Recommendation Action Required/ Assignment Date Completed A new formulation of dantrolene, Ryanodex, is available in a 250 mg per 20 mL vial, which requires further dilution with 5 mL of sterile water for injection. One vial provides a loading dose as opposed to 12 or more vials of traditional dantrolene, allowing quicker treatment of MH. The new formulation also eliminates the need to stock liter bags of sterile water. VARIZIG (varicella zoster immune globulin [human]) dilution issues reported A child was to receive 125 units of Varizig Consider adding an auxiliary label that IM. A nurse used the entire 8.5 mL of the reminds practitioners to reconstitute the supplied diluent to reconstitute the drug drug with the correct volume (1.25 mL per instead of 1.25 mL as stated in the 125 unit vial, which provides 100 units per package insert. To administer this volume, mL) and then to discard the remaining the nurse needed to give the child three IM diluent. A new liquid form of the product is injections. Subtherapeutic doses are also under development that will not require possible if the entire diluent is used and reconstitution. staff believes this provides the labeled 100 units per mL concentration (the concentration when 1.25 mL of diluent is used). Dinoprostone (PROSTIN E2) suppository confused with progesterone (17) A pregnant patient with asymptomatic uterine contractions was given a Prostin E2 vaginal suppository instead of a progesterone vaginal suppository. Both suppositories were available in the labor and delivery area. Prostin E2 suppositories are used for evacuation of uterine contents for missed abortion or intrauterine fetal death (IUFD). The patient developed contractions and delivered a 1.1 kg baby. Remove the Prostin E2 suppository from stock in patient care areas and add a warning on the screens of pharmacy and electronic prescribing systems that Prostin E2 is indicated for IUFD, uterine evacuation, and termination of pregnancy only. Barcode scanning verification at the point-of-care should be used when available. Nitroglycerin injection labeling issues during shortages October 23, 2014 ISMP MedicationSafetyAlert! QAA 5 July - September 2014 ISMP QuarterlyActionAgenda Organization Assessment No. Problem Recommendation (17) Baxter labels nitroglycerin with the mg per total volume (100 mg/250 mL) listed first, and the mcg/mL concentration (400 mcg/mL) below in parentheses, while Hospira labels nitroglycerin with the mcg/mL (100 mcg/mL) listed first, and the total mg/volume below it in parentheses. In a recent report, a nurse set an infusion pump for 200 mg/250 mL after misreading 200 mcg per mL on a container label. During shortages, draw attention to the nitroglycerin label information that matches the way staff are used to it appearing on nitroglycerin labels, and reprogram infusion pump libraries as necessary. Meanwhile, FDA is examining the differences in the labels, and ISMP is conducting a survey regarding nurses’ preferences. October 23, 2014 ISMP MedicationSafetyAlert! Action Required/ Assignment Date Completed QAA 6