Lab 1
advertisement

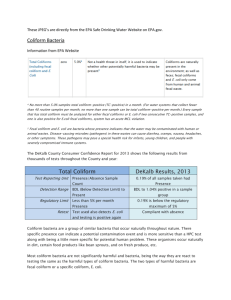
Lab 1. ENVR 421 - Environmental Health Microbiology - Spring Detection of Total and Fecal Coliform Bacteria and Escherichia coli by Broth Culture, Quantal (“Most Probable Number”) Methods: Multiple Fermentation Tube (MFT) and Defined Substrate Methods I. INTRODUCTION AND BACKGROUND Traditionally, indicator bacteria have been used to determine the possible presence and to estimate the amount of fecal contamination in water, foods and other samples. The detection of indicator bacteria is preferred over direct pathogen detection because the former are considered to be normal, non- pathogenic intestinal inhabitants that are present in feces, wastewater and other fecal wastes in much higher numbers than pathogenic microorganisms and because they are technically easier to detect and quantitate than pathogens. Present criteria and standards for the sanitary quality of water, foods and other materials with respect to fecal contamination are expressed in terms of concentrations of indicator bacteria. The most widely-used indicator bacteria in the U.S. and worldwide are the so-called total and fecal coliforms and specifically Escherichia coli, the bacterium in these groups that is most feces-specific. Although taxonomically meaningless, the word "coliform" has been used historically to represent various genera of the family Enterobacteriaceae that ferment lactose. The definition of a coliform has now been updated to include those Gram-negative bacteria that have the ability to metabolize Betagalatosides as substrates (because they possess the enzyme Beta-galactosidase). The ability to ferment lactose depends upon the possession of the enzyme beta- galactosidase as well as a galactoside permease which facilitates lactose entry into the cell. It should be emphasized that "coliforms" are actually defined in operational or functional terms based upon the media, biochemical reactions and incubation conditions used for their isolation and quantitation. None of these operational definitions detects all lactose-fermenting (or Beta-galactosidase-producing) members of the family Enterobacteriaceae and some include members of other families. From a historical perspective, the term "coliform" was probably meant to include all of the lactose fermenting species of the family Enterobacteriaceae which are commonly found in the feces of humans and the higher animals and to exclude genera of non- or slowly-lactose-fermenting bacteria, some of which are enteric pathogens in humans and animals and some of which are non-enteric pathogens and exist naturally in the environment. The ability to ferment lactose (and produce Beta-galactosidase) is thus a characteristic of considerable diagnostic importance in distinguishing among the various groups of enteric bacteria. Historically, two major operational definitions of coliforms have been distinguished: "total coliforms" and "fecal coliforms” (the latter also called “thermotolerant coliforms”). The latter group is considered a more specific indicator of fecal contamination because the former group probably includes more bacteria of non-fecal origin. Total coliforms are operationally defined in different ways in Standard Methods for the Examination of Water and Wastewater (1). The Multiple-Tube Fermentation Technique defines coliforms as "all aerobic and facultative anaerobic, gram-negative, non-spore-forming, rod-shaped bacteria that ferment lactose with gas formation within 48 hr. at 35oC." In this method the coliform bacteria are detected and quantitated by their ability to grow and produce gas in lactose-containing liquid medium under specified incubation conditions. The broth culture techniques for total coliforms actually consist of three successive steps or tests: Presumptive, Confirmed and Completed. For the Presumptive Test, dilutions of the sample are inoculated into fermentation tubes of lactose or lauryl tryptose broth and incubated at 35oC for 48 hr. For the Confirmed Test, organisms from all positive fermentation tubes (those with growth plus gas) of the Presumptive Test are transferred to fermentation tubes of brilliant green lactose bile broth and incubated at 35oC for 48 hr. Tubes showing both growth and gas are considered positive Confirmed tubes. For the Completed Test, organisms from positive Confirmed tubes are isolated in pure culture on agar plates of differential/selective media (Endo or eosin methylene blue agar) and then tested for: (1) growth and gas production in fermentation tubes of lactose or lauryl tryptose broth incubated at 35 C for 48 hr.; and a negative reaction in the Gram stain. For a positive Completed Test, the organisms must show growth plus gas production in the fermentation tubes and be Gram negative. The Completed test is rarely done in practice due to its complexity and additional time. The concentration of total coliform bacteria is estimated from the numbers of confirmed positive and negative culture tubes or other containers) that received specified and often different volumes of a water sample. This estimate is based on computing the Most Probable Number of bacteria using so-called maximum likelihood estimates and the assumption that the bacteria conform to a Poisson distribution. More on this later. Fecal Coliforms Although the total coliform group as presently operationally defined includes bacteria usually found in the feces of humans and other warm-blooded animals, some of the bacteria detected by these procedures are sometimes also found in soil (Citrobacter, Enterobacter and Klebsiella), on various plants, including grains and trees (Serratia, Klebsiella and Enterobacter) and in certain industrial wastes. Furthermore, some coliform bacteria can also be pathogenic for humans and animals when present either in the gut (e.g., various pathogenic E. coli) or in other parts of the body (e.g., Klebsiella pneumonia in the respiratory tract). In addition, organisms that are not members of the family Enterobacteriaceae and not exclusively of intestinal origin, such as members of the genus Aeromonas, may be detected in the total coliform test. For these reasons, another group, the "fecal coliforms" or “thermotolerant coliforms” has been established in an attempt to exclude those total coliforms that are non-fecal and detect only those of fecal origin. The basis for this exclusion and selection is a higher incubation temperature of 44.5OC, at which presumably coliforms of only fecal origin will grow because they are adapted to the higher temperature of the mammalian intestinal tract of about 36-37oC. Coliforms from non-fecal, environmental sources are generally incapable of growing at this elevated temperature. Thus, fecal or thermotolerant coliforms can be defined as gram-negative, non-sporeforming, rod-shaped bacteria which ferment lactose with the production of gas at 44.5oC within 24 hr. The fecal coliform test is now widely applied to surface and ground water, sewage treatment systems, biosolids (treated sludges) and general monitoring of natural waters for sanitary quality, including recreational and shellfish waters, and water quality standards have been developed for it. However, the coliform test is still used in the examination of potable waters in the USA and some other countries, because coliform bacteria of any kind are considered undesirable and are not tolerated in a finished drinking water. Today, both the total and fecal coliform tests are used in the examination of water, even though E. coli has been identified as the most feces-specific member of the coliform group. There are actually several approved Multiple Tube Fermentation Techniques for fecal coliforms and two main ones are described here: a single-step and a two-step procedure. The latter is the traditional test that has been widely used for some time, and the former is a more recent test approved for some applications. In the single-step procedure dilutions of the sample are inoculated into fermentation tubes of A-1 medium. The tubes are first incubated for 3 hr at 35oC and then transferred to a 44.5oC water bath for an additional 21 hr of incubation. Tubes showing growth plus gas are considered positive for fecal coliforms. For the two-step Multiple Tube Fermentation Technique for fecal coliforms, positive tubes from the Presumptive (total) coliform test are inoculated into fermentation tubes of EC medium and incubated at 44.5oC for 24 hr. Tubes showing growth with gas production are considered confirmed positives. Escherichia coli. Because both the total and fecal (thermotolerant) coliform tests often still detect bacteria of non-fecal origin, test have been devised to detect E. coli exclusively as the coliform bacterium most specific for fecal contamination. An early approach to this effort was the use of four biochemical tests to separate E. coli from other lactose-fermenting Enterobacteriaceae: Indole test - detects indole production from tryptophane. E. coli is positive (+); many other coliforms are negative. Methyl Red test - detects acid production in the medium; intended to distinguish between type of fermentation (mixed acid versus butylene glycol). E. coli is (+) and some other coliforms are (-). Voges-Proskauer test - detects acetoin, an intermediate in the butylene glycol pathway. Acetoin is oxidized to diacetyl under alkaline conditions in the presence of air, and when reacted with creatine, it forms a pink color. E. coli is (-) and some other coliforms are (+). Citrate utilization as sole carbon source. E. coli is (-) and many other coliforms are (+). Therefore, in this series of four tests, called the IMViC tests, E. coli is typically ++-- and Enterobacter aerogenes is typically --++. Other coliforms give different reaction patterns and are designated "intermediates". However, the IMViC reactions have been found to be imperfect in speciating E. coli, and subsequently, other approaches to E. coli and coliform speciation have been developed. Additional or alternative approaches to detecting and speciating E. coli are worth noting here. One additional approach to coliform speciation is the use of rapid, commercial, biochemical test kits designed to carry out several biochemical tests simultaneously in incubation periods of 4 to 24 hours. The results of each test are scored as (+) or (-) and assigned a number based on the relative reliability of the test. These results constitute a "code" which is compared against a data base of accumulated reaction codes for nearly all of the medically important Enterobacteriaceae. The code that is identical or closest to the organism tested is taken as its identity at the genus and species level. An alternative approach to the simultaneous detection and quantitation of coliforms and E. coli in water and other samples is the use of a single property or biochemical test in addition to lactose fermentation to definitively identify E. coli among other coliforms. One such property is the presence of the enzyme beta-glucuronidase, which is found only in E. coli and some Salmonella and Shigella. Tests for this enzyme have been developed in which a beta-glucuronidase substrate, a hydrolysable betaglucuronide compound, such as MUG (4-methylumbelliferyl-beta-D-glucoronide) is incorporated into a coliform medium. If E. coli is present, the MUG is hydrolyzed to yield a fluorogenic product (4-methylumbelliferone) whose blue fluorescence can be readily seen by shining a long wavelength UV light onto the culture. The MUG substrate can be added to coliform and fecal coliform media so that when E. coli is present and grows in the culture medium broth, the medium fluoresces blue under long wavelength UV light. Other tests for coliforms and E. coli have been devised in which the Betagalactoside substrates (for the beta-galactosidase enzyme of any coliform) and the betaglucuronide substrates (for the beta-glucuronidase activity of E. coli) can be detected because the hydrolysis products have a unique and distinguishable color. That color appears in the broth culture medium or in the bacterial colonies if the bacteria are cultured on a solid medium (one containing agar as a gelling agent, for example). Some commercially available media and test systems for E. coli and coliforms use defined substrates for beta-galactosidase and beta-glucuronidase (e.g., MUG) in a mineral salts solution to simultaneously detect and quantify coliforms and E. coli, respectively, by characteristic color changes resulting from hydrolysis of the defined substrates. These substrates are present as the only food supply for the bacteria, resulting in tests with high specificity in detecting the target bacteria, coliforms or specifically E. coli. The commercial liquid or broth culture tests are available in several formats, including multiple tubes or multiple wells. One widely is known brand of this test is “Colilert" (IDEXX Corp.). It is now widely used in bacteriological analysis of drinking water, although similar commercial tests have been developed as well, such as “Colitag” (CPI International). Similar tests based on defined chromogenic substrates detect the target bacteria as colonies on a solid medium (containing agar or pectin) or on a membrane filter, on which the colonies have a distinctive color resulting from hydrolysis of a chromogenic beta-galactoside (total or fecal coliform) or beta-glucuronide (E. coli) substrate that yields a distinctively colored hydrolysis product. One such test is Easygel, which uses pectin as the gelling agent and in which coliform bacteria grow as pinkmagenta colonies while E. coli grow as purple-blue colonies. Presence-Absence Tests for Indicator Bacteria For bacteriological analysis of drinking water, where coliforms and E. coli are supposed to be largely or always absent, respectively, testing for these organisms is often done in a "presence-absence" format using 100-ml sample volumes. Essentially, coliforms and E. coli must be absent in 100-ml volumes of drinking water, so a single 100-ml volume is examined for the presence or absence of the organisms. The test is repeated on multiple 100-ml samples on a regular basis in order to determine if coliforms and E. coli are regularly absent. II. PURPOSE To estimate the total and fecal coliform and E. coli concentrations in samples of surface water or wastewater by broth culture quantal techniques using traditional Multiple-Tube Fermentation Techniques and Defined Substrate techniques. III. PROCEDURE: A. Aseptic Technique: Use it for all operations. Use pipets and pipet aids. B. Samples and Sample Dilutions: The samples to be analyzed are one of the following: 1. Raw sewage 2. Treated sewage effluent 3. Morgan Creek water upstream of sewage effluent discharge to the creek 4. Morgan Creek water downstream of sewage effluent discharge to the creek. Dilute the sample you select serially 10-fold as directed by the instructor. Mix the sample by shaking 25 times before diluting. To make a 10-fold dilution, pipet 11 ml of sample into a 99-ml dilution blank. Seal the top of the dilution container and mix vigorously 25 times. Repeat these steps in the 10-fold dilution to make a subsequent 10-fold dilution (100-fold overall); repeat as required for further dilutions. C. Two-Step-Tube Fermentation Tube Technique for Total and Fecal Coliforms and E. coli (5 tubes per dilution): 1. Day 0: Inoculation of tubes for presumptive test: Place 25 fermentation tubes of lauryl tryptose or lactose broth in a test tube rack as groups of 5 dilutions, and mark the tubes as to their dilutions. Using a 5- or 10-ml pipet, inoculate 1-ml volumes of each sample dilution to be tested into the 5 replicate tubes. Incubate the tubes in a 35oC air incubator. 2. After 24 and 48 hr: Reading of presumptive positive tubes and inoculation of tubes for Confirmed Total and Fecal Coliform Tests and for E. coli. Gently shake the rack of tubes back and forth several times to release gas in positive tubes. Examine all tubes for the presence of growth (turbidity or cloudiness) and gas (look in the small inverted tube), and score tubes showing both growth and gas as presumptive positive. Submit all lactose broth fermentation tubes that are Presumptive positive at about 24 hr and all additional Presumptive positive tubes at 48 +/- 3 hr to the confirmed tests. Confirmed tests: inoculation of material from presumptive positive tubes into tubes of conformation media Insert a sterile wood applicator into the broth of a positive tube to a depth of >1 inch to wet the end. Transfer the organisms on the wetted end of the applicator to a fermentation tube of brilliant green lactose bile (BGLB) broth (coliform confirmatory broth) by briefly immersing the wet end of the applicator into the BGLB broth. Use the same applicator and same procedure to transfer material from the same Presumptive positive tube to a fermentation tube of EC medium containing MUG (fecal coliform-E. coli confirmatory broth). Discard the applicator. For each positive Presumptive tube, use a fresh applicator stick and repeat these steps above. Incubate the BGLB broth tubes in a 35oC incubator and the EC-MUG tubes in a 44.5oC water bath. D. Reading Confirmed Tubes After 24 (+/- 4) and 48 (+/- 4) hours of incubation: Examine BGLB tubes for growth plus gas. Tubes with growth plus gas are confirmed positive. If a tube is negative after 24 hours, continue incubation to 48 hours and read again for positivity (growth plus gas). Record Examine the EC tubes at 24 +/- 2 hr for growth plus gas. Tubes showing both are scored as Confirmed positive for fecal coliforms. Examine the positive EC tubes under a long wavelength UV light. Tubes showing bluish fluorescence under long wavelength UV light (in addition to growth plus gas) are scored as positives for E. coli. Record the results of all BGLB (total coliform) and EC-MUG (fecal coliform and E. coli) tubes as confirmed positive or as negative. 4. Calculation of Most Probable Number Calculate the total and fecal coliform and E. coli densities of your sample from the number of positive and negative tubes of three sample dilutions according to the procedures described in Standard Methods for The Examination of Water and Wastewater and below. Bring these results to the next scheduled laboratory period. D. Detection of Coliforms and E. coli using a defined substrate “Colilert” quantal method. A. If your sample is waste water, dilute the sample serially 10-fold according to directions from the instructor. If your sample is treated sewage effluent or Morgan Creek water is does not need to be diluted. Mix the sample by shaking 25 times before diluting. To make 10-fold dilutions, pipet 11 ml of sample into a 99-ml dilution blank. Seal the top of the dilution container and mix vigorously 25 times. Perform the “Colilert” method in Quantitrays according to the manufacturer’s instructions and those of the class instructor 1. Briefly, dispense a 100 ml sample into a sterile bottle and aseptically add the powdered contents of a Colilert packet to the sample. Close the sample container and swirl to dissolve the medium in the sample. 2. Open a Quantitray by peeling back a portion of the seal as per manufactures instructions. Pour the 100 ml sample containing dissolved Colilert medium into the Quantitray and re-seal it according to the manufacturer’s instruction (using the special sealing device). 3. Incubate the Quantitry at 35oC for the specified time period and then remove to score for positive results in each well. 4. Examine each well and score as positive for total coliforms if it has a bright yellow color indicative of ONPG hydrolysis. 5. Shine a long wavelength UV light on the Quantitray and score each well as positive for E. coli if it fluoresces with a blue color indicative of MUG hydrolysis. 6. Record these results for positive and negative wells for total coliforms (yellow color) and E. coli (blue fluorescence) and bring to the next lab class to compute the MPN concentration using the manufacturer’s MPN table. RESULTS AND QUESTIONS Multiple Fermentation Tube Method Compute and record the Most Probable Number of total and fecal coliforms and E. coli per 100 ml using the data from the confirmed tubes (BGLB and EC-MUG). Also record the upper and lower 95% confidence limits for these MPN values. The MPNs and confidence limits are found in the table for three dilutions and five tubes per dilution in Standard Methods for the Examination of Water and Wastewater. Note that the MPN table in Standard Methods is set up for a dilution series of 10, 1 and 0.1 ml sample volumes. The sample volumes examined in your samples are smaller( 1, 0.1 and 0.01 ml or even less), and therefore, the MPN values in the table have to be multiplied by factors of 10 (or more for some samples) to correct for the difference in sample volumes analyzed. Colilert Quantitray MPN Results Compute and record the Most Probable Number and corresponding 95% confidence interval of total coliforms and E. coli per 100 ml based on the Colilert Quantitray results for total coliforms (numbers of yellow colored wells) and E. coli (numbers of blue fluorescing wells) using the MPN table provided by the manufacturer. If you diluted a wastewater sample prior to analysis, correct the MPN results for the additional dilution factor of this sample. Questions on Traditional Multiple Fermentation Tube and Colilert Results 1. For each method (multiple fermentation tube and Colilert) how well do the results for the replicate analyses of each sample agree? Specifically, are they identical? If not, does the MPN value for one replicate fall within the 95% confidence limit of the other replicate? 2. For each sample, compare the traditional multiple fermentation tube MPN concentrations of total and fecal coliform and E. coli. Which indicator concentration is highest? Which is lowest? Are these results consistent with what you would expect, based upon the definitions of each indicator and the expected relationships among them? 3. For each sample, compare the Colilert MPN concentrations of total coliforms and E. coli. Which indicator concentration is highest? Which is lowest? Are these results consistent with what you would expect, based upon the definitions of each indicator and the expected relationships among them? 4. For the corresponding samples, compare the MPN concentrations of total coliforms and also E. coli based on traditional multiple fermentation tube MPN results and Colilert Quantitray MPN results. How well do the results of these two methods agree? Are they identical? Does the MPN value of one method fall within the 95% confidence interval of the other method? 5. Recall that the four samples analyzed were: raw sewage, treated sewage effluent, Morgan Creek (the receiving water) upstream of the sewage effluent discharge, and Morgan Creek downstream of the sewage effluent discharge. 6. Compare the levels of the three indicators in the four samples. Which sample had the highest levels of indicators? Which the lowest? 7. Considering that the raw sewage was treated and then discharged to Morgan Creek, what is the impact of the sewage effluent discharge on the concentrations of indicator bacteria in Morgan Creek? 8. How effective was the sewage treatment plant in reducing the concentrations of indicator bacteria in raw sewage. (Sewage treatment at this plant consists of (i) primary settling, (ii) secondary (biological) treatment and (iii) chlorine disinfection of the secondary-treated effluent.). Specifically, calculate the % (or log10) reductions of total and fecal coliforms and E. coli by dividing the concentrations of these bacteria in the treated effluent by the concentration in the raw sewage, then subtracting from 1 and multiplying by 100. [(Concentration in effluent/concentration in raw sewage) - 1] x 100 = % reduction. 9. Also, compute the log10 concentrations of each group of indicator bacteria in the raw sewage and treated effluent, and subtract the latter from the former to compute the log10 reduction by sewage treatment. Log10 concentration in raw sewage - log10 concentration in treated effluent = log10 reduction.