Chlorophyll writeup
advertisement

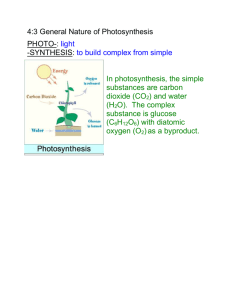
Andy Young, Chris Walthers, Fei Fei Xue, Emmy Wilde 2 December 2004 Chlorophyll Final Model Project Writeup Chlorophyll, chemically represented as C55H70O6N4Mg, is the key component in allowing plant cells undergo photosynthesis. Located in the chloroplast, chlorophyll is a complex molecule that exists as either Chlorophyll A or Chlorophyll B. Chlorophyll absorbs various wavelengths of light that correspond to different colors on the light spectrum. It primarily absorbs reds and blues, which gives Chlorophyll its green color. Chlorophyll absorbs the sun’s energy and transfers it into chemical energy using the process of photosynthesis: CO2 + H2O + light energy → (CH2O)n + O2 Chlorophyll is of paramount importance because it is the basis of all energy creation for biological systems. Within the process of photosynthesis, chlorophyll plays two important roles: it is both the energy gathering mechanism of the cell, and a conduit that shifts the electrons within the cell to the necessary site for use. Both Chlorophyll A and Chlorophyll B are important; however Chlorophyll A is the primary type necessary for photosynthesis to occur. Both types of chlorophyll can gather energy and gain electrons only with certain input. However, because only Chlorophyll A is able to pass the electrons to the primary electron acceptor, Chlorophyll B must first shift the energy to Chlorophyll A. Since the primary electron acceptor is the point where the electrons are converted into glucose, a usable form of energy, this passing of the electrons from Chlorophyll B to A is vital. Within the cell, chlorophyll is located in the organelle chloroplast, and is anchored by a hydrocarbon chain. Because the chloroplast is such an important structure, it is well protected within the cell. Chloroplasts have two membranes that surround and protect it. Within the structure of chloroplast lies the granum, a large chain of thylakoids. The chlorophyll is located on the outside of the granum, where, depending on the number of membranes, it is able to absorb more light. By definition, chlorophyll is a photosynthetic pigment. Structurally, the molecule of chlorophyll is designed for a central light intake based around a chlorin ring, which is centered on a magnesium ion. This area of a large magnesium atom surrounded by nitrogen and various hydrocarbons is the site of absorption for the UV light. Branching out of this central ring are various hydrocarbons, primarily CH2 and CH3. To anchor the entire structure into the cell, chlorophyll uses a long hydrocarbon tail. On the nanoscale, the structure of chlorophyll is quite large. Although we were unable to find data reporting the specific size of chlorophyll, we discovered some descriptions of the molecule, the best was found in C.J. Horn’s article “From Photons to Chlorophyll: Some Observations Regarding Color in the Plant World.” Horn describes chloroplast as unfathomably small, but also says that “one square millimeter at the middle of a leaf has 500,000 chloroplasts.” To compare this figure to another subject that we’ve studied, chloroplast, excluding its tail, is very small relative to a protein. Although we couldn’t find a specific entire size of chlorophyll, we are able to describe the sizes of its components. The respective radii of the atoms involved are: Magnesium 150 pm, Hydrogen 25 pm, Carbon 70pm, Nitrogen 65pm, and Oxygen 60pm. To give another idea of the small size of chlorophyll, the entire weight of all these parts is 906 grams per mole. Focusing only on the light absorbing area and ignoring the tail, the size of chlorophyll can be viewed on the nanoscale. Our model of chlorophyll A doesn’t include the tail so it consists of 77 separate atoms of Magnesium, Nitrogen, Oxygen, Hydrogen, and Carbon. The implications of chlorophyll relate to the idea of energy sources on the nano scale level. We can learn from nature’s structure to gather and create energy on the basic scale. These concepts and ideas can be transferred to man-made machines that require solar power or other sources of energy that are based on exterior sources of power. The storage of electrons that is utilized in chlorophyll can also be used as a potential model for fuel cells, photovoltaic solar cells, or LED’s. These exist on the macro scale right now, but enough technology exists to potentially lead to an artificial process similar to photosynthesis. Essentially, the main implication of chlorophyll is learning about how nature and evolution have created, on the molecular scale, the most efficient method of gathering energy. On the nanoscale, our model represents a chlorophyll A molecule without the anchor. This includes the chlorin ring based around the magnesium atom, plus the surrounding nitrogen, oxygen, and hydrocarbons. We chose to build the model out of Styrofoam balls and toothpicks. Each type of atom was represented by a different color of Styrofoam ball (red=hydrogen, yellow=nitrogen, silver=magnesium, blue=oxygen, and black=carbon). We also had various sizes of Styrofoam balls to represent the various sizes of each component atom. The toothpicks used were of two colors, yellow for basic bonds, and two red toothpicks for double bonds. To start, we painted all the balls with a thin layer of glue mixed with water so that when we spray painted them, they would not dissolve. We then coated them in various colors to represent the different atoms in the molecule. Then we picked out the parts of the chlorin ring and coated them with a translucent glow in the dark paint. These glow in the dark atoms represent where the photons emitted by sunlight are absorbed and electrons are gathered. After the Styrofoam balls dried, we began to assemble our model. We connected the balls with toothpicks separated by about half an inch or so, to keep the model geometrically possible on our macro level. Super glue was used to strengthen the bonds to preserve the model. We chose to build the model out of these materials for a few reasons. First they were readily available and inexpensive. Also all of these materials are lightweight, but sturdy enough to be preserved for a few years. Also we had various colors and sizes at our disposal so that we could better create our model to represent varying types and sizes of the atoms. The main problem we encountered while building the model was when we were initially spray painting. The spray paint dissolved parts of the Styrofoam balls we were working with. In order to solve this problem, we coated each of the balls with a thin coating of glue before spray painting them. The diagram that we based our model on is depicted in the write-up, and pictures of our model follow: Works Cited “Chlorophyll”. Wikipedia, the Free Encyclopedia. 24 November 2004. 28 December 2004 <http://www.ch.ic.ac.uk/local/projects/steer/chloro.htm>. Horn, C.J. “From Photons to Chlorophyll”. Institute for Creation Research. 28 December 2004 <http://www.icr.org/goodsci/bot-9711.htm>. “Introduction to Photovoltaic (solar cell) systems”. Clean Energy Basics. 28 December 2004 <http://www.nrel.gov/clean_energy/photovoltaic.html>. Kimball, John. "Kimball's Biology Pages." Chlorophylls and Carotenoids. 02 Dec. 2004 <http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/Chlorophyll.html>. Rushin. “Compound”. Cary Academy. 28 December 2004 <http://web1.caryacademy.org/chemistry/rushin/StudentProjects/CompoundWe>. Steer, James. “Structure and Reactions of Chlorophyll”. 28 December 2004 <http://www.ch.ic.ac.uk/local/projects/steer/chloro.htm>. Webelement Organization. 28 December 2004 <http://www.webelements.com/webelements/properties/>.