Lesson 1
advertisement

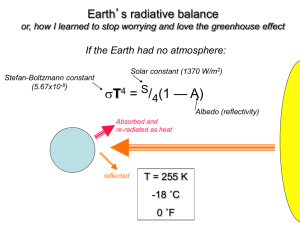
Lesson 1 Climate factor: Greenhouse Gases At a Glance Focus: Students use an experiment with the greenhouse gas water vapor to observe the greenhouse gas effect, gain an appreciation for the importance of greenhouse gases in warming the Earth, and learn how levels of greenhouse gases have changed through time. Major Concepts: Greenhouse gases help regulate the Earth’s climate – they are very important for life on Earth! CO2 is an important greenhouse gas that has a long correlation with Earth’s temperature through glacial and interglacial periods. Humans have increased the amount of CO2 in the atmosphere due to burning of fossil fuels and land use change, which plays a major role in driving of current climate change. Prerequisite Knowledge: Students should be familiar with at least some factors that regulate the global climate system and perhaps have an idea of the Earth’s energy balance. Students should be able to make hypotheses and predictions and plot data on a graph. Wyoming Science Standards Addressed: 8.2.2 Students use inquiry to conduct scientific investigations. Ask questions that lead to conducting an investigation Collect, organize, and analyze and appropriately represent data Draw conclusions based on evidence and make connections to applied scientific concepts Clearly and accurately communicate the result of the investigation 8.1.9 Students systematize the Earth’s history in terms of geologic evidence, comparing past and present Earth processes and identifying catastrophic events and fossil evidence. 11.1.7 Geochemical Cycles: Students describe the Earth as a closed system and demonstrate a conceptual understanding of the following systems: geosphere, hydrosphere, atmosphere, and biosphere. Students explain the role of energy in each of these systems, such as weather patterns, global climate, weathering and plate tectonics. Introduction This is taken directly from a background essay found at Teacher’s Domain: “Global Warming: The Physics of the Greenhouse Effect” http://www.teachersdomain.org/resources/phy03/sci/phys/matter/greenhouse2/index.html Scientific evidence has convinced most experts that there is a connection between global warming and human activities. Since the start of the Industrial Revolution, the average temperature of the planet has increased by slightly less than one degree Celsius to its present level of about 16°C (60°F). This seemingly insignificant change represents a fairly rapid warming trend. According to the UN-sanctioned Intergovernmental Panel on Climate Change, by the end of the twenty-first century, Earth's inhabitants will be facing temperatures that are at least two degrees Celsius — and possibly as much as six degrees — warmer. To understand how human activities might cause global warming, we must first explore why our planet is warm in the first place. As solar radiation from the Sun reaches Earth's surface, some of it is reflected back into space and some of it is absorbed. The absorbed energy warms Earth's surface, which in turn radiates the energy back toward space. Molecules in the atmosphere, known as greenhouse gases, absorb some of this outgoing energy and radiate a portion of it back to Earth's surface. Through these two actions, they slow the escape of heat and keep Earth warmer than it would be otherwise. When the concentration of greenhouse gases in the atmosphere increases, the atmosphere is capable of absorbing more energy. As a result, the planet warms up — until it reaches the temperature at which it again radiates just enough energy to keep the temperature stable. The higher the concentration of greenhouse gases in the atmosphere, the warmer the planet becomes before it reaches the point at which it radiates all the energy it receives and the temperature again stabilizes. Consequently, an increase in greenhouse gas concentrations — whether as the result of natural causes or human activities — causes the average global temperature to rise. Scientists have documented changes in atmospheric carbon dioxide (CO2), one of the four most important greenhouse gases, over the past 400 million years. Their findings show a strong correlation between CO2 concentrations and global climate over geological history. Of course, atmospheric and climate changes in Earth's distant past have been unrelated to human activities. Most peaks in atmospheric carbon dioxide concentrations, for example, are attributed to the movement of carbon stored on Earth's surface — on land and in oceans — into the atmosphere through natural processes such as volcanic eruptions. However, scientists have found no such natural causes for the dramatic increase in CO2 observed since the Industrial Revolution. Instead, human activities are the main source of CO2 and other greenhouse gases. Burning wood and fossil fuels such as gas, coal, and oil contributes carbon dioxide; livestock and coal production add methane; and agricultural and industrial processes add nitrous oxide. While oceans and land plants absorb a portion of these gases, the rest accumulate in the atmosphere, where they strengthen the greenhouse effect and cause the average global temperature to increase. To learn more about evidence of regular, extreme climate change throughout Earth's distant past, check out Climate Change, Greenland Ice Sheet Project 2: A Record of Climate Change, and Natural Climate Change in Djibouti, Africa. To learn more about the role CO2 plays in Earth's temperature, check out Global Warming: Carbon Dioxide and the Greenhouse Effect. To learn more about evidence suggesting a link between human activities and global warming, check out CO2 Concentrations at Mauna Loa Observatory, Hawaiʻ i and Earth System: Ice and Global Warming. Materials & Preparation You will need the following materials to conduct these activities: Overheads of graphs, or capability to show computer simulations three thermometers two clear glass jars that will fit over the thermometers heat lamp paper towels and glass of water Large graph paper with the axes pre-drawn and posted in the front of the room One clock or stopwatch to time measurement intervals Procedure 1. Begin by asking the class what they know about greenhouse gases. After students explain what they know, show a demonstration of the greenhouse effect with either a computer simulation or overhead diagram. Be sure students can understand the concept of an Energy balance (energy is not created or destroyed, but can change forms, be reflected, absorbed, or reemitted) and identify where short wave and long wave radiation fit in. 2. Have students name some of the greenhouse gases: CO2, H2O vapor, CH4, N2O Ask the question: What would the Earth be like if we did not have these gases in our atmosphere? (Greenhouse effect warms the Earth approximately 33 degrees Celsius, that’s 91.4 degrees Fahrenheit! 3. Tell the students: Now we will begin an experiment to test the greenhouse gas effect. Be sure that the thermometers all read the same room temperature. Cover two thermometers with glass jars, leaving one thermometer uncovered. Students should place a wet paper towel inside one of the two jars. Use water at room temperature to wet the paper towel. (In this experiment, the water vapor will act like a greenhouse gas and increase the temperature in the jar with the wet paper towel even more than the temperature in the dry jar.) Continue with the lesson, but have students periodically check all three thermometers and record the temperature and time on the graph at the front of the room. 3. Ask the students if they can recall any time in Earth’s history where organisms shaped the atmosphere. How do organisms shape the atmosphere today? Remind students that the original Earth’s atmosphere did not have oxygen in it. It is only the result of cyanobacteria (photosynthetic bacteria) that the Earth’s atmosphere became oxygenated. 4. Discuss carbon dioxide: how does this cycle into and out of the atmosphere? Plants take up CO2 during the growing season, and decomposition and respiration put CO2 back into the atmosphere. Other sources of CO2 are forest fires, burning of fossil fuels, and exchange with the oceans (both source and sink of CO2). Show the graph of CO2 with oscillations corresponding with growing season CO2 sinks from Mona Loa and have students identify where these growing seasons occur. Suggest the thought question: Would this graph look different if the measurements were taken somewhere along the equator? (As long as there are growing season changes: wet and dry season, or summer and winter, there will be changes, but if a place is fairly aseasonal, these changes will be less dramatic) 5. Put up the graph of the history of CO2 during glacial and interglacial history. Discuss the patterns, and how glacial periods correspond with low CO2 in the atmosphere… what was the Earth’s climate like during these times? Now point to interglacial periods… what was the Earth’s climate like then? 6. Put up the graph of recent CO2 concentrations in the atmosphere… what happened around 1850 that could make the CO2 concentration begin to rise? Does this match the pattern with average temperatures as well? Interesting fact: Many cities now monitor the composition of atmospheric gases throughout the year, and peaks can be seen during rush hour traffic, and even on weekends in the summer when many people are outside mowing their lawns! These observations have led some states, like CA, to push for tougher emissions laws on cars and small engines to improve air quality and try to decrease greenhouse gas emissions because of global warming. 7. What does the graph look like from the greenhouse gas experiment? Was it different for the two jars? How did the temperature change over time? **This lesson was adapted from a lesson on climate change from Teacher’s Domain (Global Climate Change: Understanding the Greenhouse Effect), here are some great references that they provide with background and other great video clips and visuals. Lots of great graphs here: http://en.wikipedia.org/wiki/User:Dragons_flight/Images Climate Change: http://www.teachersdomain.org/resources/ess05/sci/ess/watcyc/climatechange/index.html Greenland Ice Sheet Project 2: A Record of Climate Change: http://www.teachersdomain.org/resources/ess05/sci/ess/watcyc/greenland/index.html Natural Climate Change in Djibouti, Africa: http://www.teachersdomain.org/resources/ess05/sci/ess/watcyc/naturalchange/index.html Global Warming: Carbon Dioxide and the Greenhouse Effect: http://www.teachersdomain.org/resources/phy03/sci/ess/watcyc/co2/index.html CO2 Concentrations at Mauna Loa Observatory, Hawaiʻ i: http://www.teachersdomain.org/resources/ess05/sci/ess/watcyc/maunaloadata/index.html Earth System: Ice and Global Warming: http://www.teachersdomain.org/resources/ess05/sci/ess/earthsys/esglaciers/index.html Or: