Set 8
advertisement

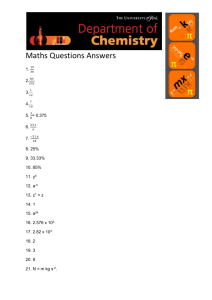
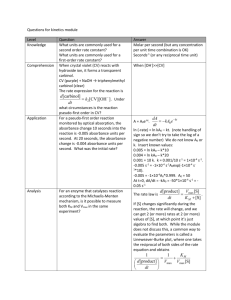
CHM 235 Quantitative Analysis Spring 2007 Dr. S.A. Skrabal SOLUTIONS TO PROBLEM SET 8 Spectrophotometric analysis 20 March 2007 NOTE: THERE ARE ADDITIONAL QUESTIONS ON THE BACK. 1. Understand the qualitative and quantitative concepts about light and spectroscopy asked about in textbook questions 18-1, 18-2, 18-3, 18-4, 18-6, 18-7, 18-21, 18-22, 18-23, and 18-24. 18-1. (a) double (b) halve (c) double 18-2. (a) E = h = (6.626 x 10-34 J s) ; = c/ = (2.998 x 108 m s-1)/650 x 10-9 m) = 4.61 x 1014 s-1 ; E = (6.626 x 10-34 J s)(4.61 x 1014 s-1) = 3.05 x 10-19 J (per photon) ; (3.05 x 10-19 J photon-1)(6.022 x 1023 photon mol-1)(1 kJ/1000 J) = 184 kJ mol-1 (b) = c/ = (2.998 x 108 m s-1)/400 x 10-9 m) = 7.50 x 1014 s-1 ; E = h = (6.626 x 10-34 J s)(7.50 x 1014 s-1)(6.022 x 1023 mol-1)(1 kJ/1000 J) = 299 kJ mol-1 8 18-3. = c/ m s-1)/(562 x 10-9 m) = 5.33 x 1014 s-1 or Hz -9 (562 x 10 m)(100 cm/m) = 5.62 x 10-5 cm ; wavenumber = 1/5.62 x 10-5 cm = 1.78 x 104 cm-1 E = h = (6.626 x 10-34 J s)(5.33 x 1014 s-1) = 3.53 x 10-19 J (per photon) (3.53 x 10-19 J photon-1)(6.022 x 1023 photons mol-1)(1 kJ/1000 J) = 213 kJ mol-1 18-4. Microwave---rotation; infrared---vibration; visible---electronic excitation; UV---electronic excitation 18-6. Transmittance---light transmitted through sample without absorption. Absorbance---light absorbed by sample. Molar absorptivity is amount of light absorbance (at a particular wavelength) per unit concentration of absorbing species per unit path length. Relationship between A and T is A = -log T. Absorbance is directly proportional to concentration through Beer’s Law; A = bc. 18-7. Absorption spectrum is plot of absorbance vs. wavelength. It shows how effectively light is absorbed by a chromophoric sample as a function of wavelength. 18-21. Phosphorescence occurs at a longer wavelength (lower energy) than fluorescence. Sketch can be similar to Fig. 18-20, but add another peak at longer wavelength that is mirror image of absorption. 18-22. Luminescence is a term describing light emission following excitation of a molecule. Chemiluminescence is light emission resulting from a chemical reaction. 18-23. Phosphorescence occurs at a longer wavelength (lower energy) than fluorescence. Sketch can be similar to Fig. 18-21, but add peaks for phosphorescence (absorption and emission), that are are mirror images. 18-24. A fluorescence excitation spectrum is a plot of light absorbed as a function of wavelength (and thus resembles an absorption spectrum) whereas a fluorescence emission spectrum is a plot of light emitted as a function of wavelength. 2. Titanium reacts with hydrogen peroxide in 1 M H2SO4 to form a yellow complex that absorbs strongly at 415 nm. (A) If a 2.02 x 10-5 M solution transmits 67.5% of the light in a 1.00 cm cell, what would be the absorbance of this solution? (B) What would be the absorbance of a 3.970 x 10-5 M solution of the complex if measured in a 1.00 cm cell? (Note: You do not need for this; remember Beer’s Law states that absorbance is directly proportional to concentration.) (A) %T = 67.5% ; T = 0.675 ; A = - log T ; A = - log (0.675) = 0.171 (B) Since A is directly proportional to C, you can set up proportionality equation: A1/C1 = A2/C2 ; (0.171/2.02 x 10-5 M) = (A2/3.970 x 10-5 M) ; A2 = 0.336 3. Using the method described in question 2, a sample containing 35.0 mg/L of Ti gave an absorbance of 0.369 when measured at 415 nm in a 1.00 cm cell. A blank, measured in the same way as the sample, gave an absorbance of 0.003. (A) What is 415 nm(in units of M-1 cm-1) of this complex when measured using a 1.00 cm cell? (B) What is the percent transmittance of this sample when measured using a 1.00 cm cell? (A) (35.0 mg Ti/L)(1 g/1000 mg)(1 mol Ti/47.87 g Ti) = 7.31 x 10-4 mol Ti/L Corrected absorbance = 0.369 – 0.003 = 0.366 A = εbc ; ε = A/bc = 0.366/(1.00 cm)(7.31 x 10-4 mol Ti/L) = 501 M-1 cm-1 (B) A = - log T ; 0.366 = - log T ; 10-0.366 = T = 0.431 ; %T = 43.1% 4. A solution containing the Fe[II](ferrozine)34- complex at a concentration of 0.998 µM had an absorbance of 0.262 when measured at 562 nm in a 10.0 cm cell. (A) What is the percent transmittance for this complex at 562 nm in a 10.0 cm cell? (B) What is 562 nm for this complex when measured using a 10.0 cm cell? (A) A = - log T ; 0.262 = - log T ; T = 10-0.262 = 0.547 ; %T = 54.7% (B) = A/bc = 0.262/(10.0 cm)(0.998 x 10-6 M) = 2.63 x 104 M-1 cm-1 5. Manganese can be determined spectrophotometrically by reacting it with the organic reagent formaldoxime in basic solution. A purple complex is formed with a maximum absorbance at 450 nm. A 25.00 mL aliquot of a waste effluent was treated with formaldoxime. A reagent blank, along with standard solutions containing known concentrations of Mn were also prepared and measured at 450 nm in a 10.00 cm cell. The absorbance of the unknown solution was measured in the same way. The absorbances of the knowns and unknown are shown in the table below. Concentration (mg/L) 0 15.0 30.0 60.0 90.0 unknown Absorbance 0.002 0.027 0.050 0.095 0.143 0.100 The following equation resulted from a regression analysis of the standards and their blank-corrected absorbances: Absorbance = 1.53 x 10-3 • [Mn (mg/L)] + 0.00088 (A) What is the concentration (in mg/L and M) of Mn in the effluent sample? (B) Use the regression equation to calculate the molar absorptivity, 450 nm, (in M-1 cm-1) of the Mn-formaldoxime complex. The stoichiometry between Mn and Mn-formaldoxime is 1:1. (A) Conc. = (abs – 0.00088) / 1.53 x 10-3 Corrected absorbance = 0.100 – 0.002 = 0.098 Conc. = (0.098 – 0.00088)/1.53 x 10-3 = 63.5 mg/L (63.5 mg Mn/L)(1 g/1000 mg)(1 mol Mn/54.94 g Mn) = 1.16 x 10-3 M (B) m = 450 nm b = 1.53 x 10-3 L mg-1 Convert the units of the slope from L mg-1 to L mol-1: (1.53 x 10-3 L/mg)(1000 mg/g)(54.94 g Mn/mol Mn)(1 mol Mn/1 mol Mn-formaldoxime) = 84.1 L mol-1 450 nm = m/b = 84.1 L mol-1/10.00 cm = 8.41 L mol-1 cm-1 or 8.41 M-1 cm-1. 6. The drug tolbutamine (FW = 270 g/mol) has a molar absorptivity of 703 M-1 cm-1 at a wavelength of 262 nm (which is in the UV region of the EMR spectrum). One tablet of this drug is dissolved in H2O and diluted to a volume of 2.00 L. If the solution exhibits an absorbance of 0.687 in a 1.00 cm cell, how many grams of tolbutamine are contained in the tablet? C = A/b = 0.687/(703 M-1 cm-1)(1.00 cm) = 9.77 x 10-4 mol tolbutamine/L (9.77 x 10-4 mol tolb./L)(2.00 L)(270 g tolb./mol tolb.) = 0.528 g 7. Another spectrophotometric method for manganese involves oxidizing the Mn in a sample with a strong oxidizing reagent to convert it to the deep purple permanganate ion (MnO4-). The absorbance of the purple solution is measured at 520 nm. A series of Mn standards ranging in concentrations from 0 to 15.0 mg L-1 was treated as described above and analyzed spectrophotometrically. The resulting regression equation for these standards was: Absorbance = 0.04294 (conc. in mg L-1) - 0.0022. A Mn-containing mineral sample weighing 0.1197 g was completely dissolved, transferred to a 100.0 mL volumetric flask, treated to convert the Mn to MnO4-, diluted to volume, and measured at 520 nm in a 1.00 cm cell. The solution gave an absorbance of 0.499. A blank gave an absorbance of 0.003. (A) What is the Mn concentration in the 100.0 mL flask? (B) What is the weight percent Mn in the mineral sample? (A) Corrected absorbance = 0.499 – 0.003 = 0.496 Conc. = (0.496 – (-0.0022))/0.04294 = 11.6 mg/L (B) (11.6 mg Mn/L)(0.1000 L)(1 g/1000 mg)(100/0.1197) = 0.969% 8. A calibration curve for blank-corrected chemiluminescence (CL) signals for a series of Cu standards in seawater ranging in concentration from 0 to 7.2 nM yielded a regression equation of CL = 418.4 [Cu] - 39.5, where CL is unitless and [Cu] is in nM. A blank and seawater sample were analyzed. The blank CL signal was 2.05 and the sample CL signal was 888. What is the Cu concentration (in nM and ppb) of the seawater sample? Corrected CL signal = 888 – 2.05 = 886 Conc. = ((886 – (-39.5)/418.4 = 2.21 nM (= 2.21 x 10-9 M) (2.21 x 10-9 mol Cu/L)(63.55 g Cu/mol Cu)(106 µg/g) = 0.140 µg/L or ppb