Carboxylic Acids
advertisement

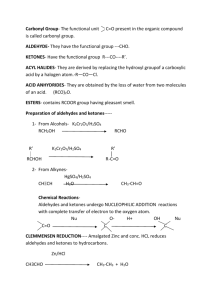
Carboxylic Acid And their Derivatives Formation of Carboxylic acids: Hydrolysis of Nitriles Hydrolysis of α-hydroxynitriles : OH OH H3O+ R — C — CN Ketones/Aldehydes + HCN R — C — COOH heat R/H Acid hydrolysis : H3O+ SN1 RX + CN- RCOOH + NH4+ RCN reflux Alkali hydrolysis : OH-/H2O SN1 RX + CN- RCOO- + NH3 RCN reflux Oxidation of Primary Alcohols and Aldehydes (1)KMnO4 RCH2OH RCOOH (2)H3O+ (1)K2Cr2O7 RCHO RCOOH (2)H3O+ Oxidation of Alkylbenzenes KMnO4/H+(aq) R R’COOH heat R/H If R contains more than one carbon skeleton , oxidation only occurs at the carbon bonding to the ring to form benzoic acid and CO2(g). but 2-methyl-2-phenylpropane is resistant to side chain oxidation. Oxidation of Methyl Ketones and some Alcohol O OH O ∥ ∥ R — C — CH3 /R — C — CH3 + 3I2 + 3OH - R — C — CH3/CI3 + 3I- + 3H2O OHO ∥ — C —O- + CHI3 (one carbon less ) Reactions of Carboxylic Acids Acidity of carboxylic acids Order of acidity : RCOOH > —OH > ROH We can think about the following factors : 1. The strength of the H-A bond 2. The electronegativity of A and , 3. Factors stabilizing its conjugate base/acid anion with respect to HA. Influence of substituents on acidity Electron withdrawing groups can increase acid strength by weakening the OH bond and stabilizing the acid anion. The positive inductive effect of E-groups is very small through more than two or three carbon-carbon bonds. Electron donating groups reduce the partially positive charge of carboxyl carbon atom , thus strengthening the O—H bond => not easily break. There will be intensified negative charge of O- and destabilized acid anion when dissociation of acids occurs. Formation of Salts Most carboxylic acids displace CO2(g) from carbonates and hydrogencarbonates but phenols do not, so we can use this test to distinguish them. Reduction with Lithium Aluminiumhydrate (1)LiAlH4/ ether RCOOH RCH2OH(Primary alcohol ) (2)H3O+ The C═C,C≡C, and phenyl parts in unsturated acids are unaffected. Conversion to Acid Derivatives (1) Conversion to acyl chlorides SOCl2 RCOCl(intermediate b.p.)+SO2+HCl or RCOOH + PCl5 RCOCl(high b.p.)+POCl3+HCl or PCl3 RCOCl(low b.p.)+H3PO3 (2) Conversion to acid anhydrides pyridine RCOOH + R’COCl O O ∥ ∥ RC — O — CR’ + HCl (3) Conversion to amides O excess RCOOH RCOOH + NH3 RCOO ∥ RC — NH2 + H2O - NH4+ ? , reflux (4) Conversion to esters H3O+ RCOOH + R’OH RCOOR’ + H2O reflux The yield of ester can be enhanced by : (1) increasing the amount of carboxylic acid or alcohol or (2) increasing the amount of inorganic acid used for catalysis. Reaction of Acyl Chlorides: (1)Hydrolysis with water O ∥ rapid CH3— C — Cl + H2O(cold) CH3COOH + HCl (2)Ester formation with alcohol O O ∥ ∥ R — C — Cl + R’OH RC — OR’ + HCl If R is aromatic alkane , a base catalyst is required, e.g. OH- . (3)Amide formation with ammonia and amines O O ∥ ∥ low temp. R— C — Cl + 2NH3(in excess) RC — NH2 + NH4Cl O O ∥ ∥ NaOH R— C — Cl + RNH2 RC — NH2R + NaCl +H2O (4)Anhydride formation O O O ∥ ∥ ∥ R— C — Cl + R’COO-Na+ RC — O — CR’ + NaCl Reaction of Acid Anhydrides (1)Hydrolysis with water slowly (RCO)2O + H2O RCOOH (2)Ester formation with alcohol (RCO)2O + R’OH RCOOR’ + RCOOH Δ If phenol is used , alkaline medium is required to provide a powerful nucleophile — phenoxide ion. (2)Amide formation with ammonia and amines RCONH2 + RCOO-NH4+ (RCO)2O + 2NH3 (RCO)2O + 2R’NH2 RCONHR’ + RCOO-R’NH3+ Reaction of Esters (1)Hydrolysis of ester OH-/reflux RCOO- + R’OH RCOOR’ + H2O H3O+/reflux RCOOH + R’OH Alkaline hydrolysis is much faster than acid catalyzed hydrolysis as the equilibrium in alkaline hydrolysis lies almost to the right. (2)Reduction to alcohol with lithium aluminiumhydrate (1)LiAlH4/ether RCOOR’ RCH2OH + R’OH (2)H3O+ Reaction of amides (1)Hydrolysis reflux RCONH2 + OH- RCOO- + NH3 reflux RCONH2 + H3O+ RCOOH + NH4+ (2)Dehydration — nitrile formation O ∥ P2O5 , Δ —C—NH2 C≡N + H2O -H2O (1)Hofmann degradation CH3CH2CONH2 + 4KOH + Br2 CH3CH2NH2 + K2CO3 + 2KBr + 2H2O one carbon less (2)Reduction to amines with lithium aluminiumhydrate O ∥ LiAlH4 R — C — NH2 RCH2NH2 in ether Some specimen results of the investigation of the reactions of ethanoic acid Reaction Observations pH of aqueous solution Orange-red-pH3-4 Reaction with sodium hydrogencarbonate solution Reaction with sodium Reaction with phosphorus pentachloride(PCl5) Reaction with 2,4-dinitrophenylhydrazine Triodomethane reaction Gas evolved which turns limewater milky—CO2(g) Gas evolved which popped with a lighted splint—H2(g) Steamy gas evolved which gave white fumes with ammonia—HCl(g) No change No change