nph12981-sup-0003-NotesS1-S3
advertisement

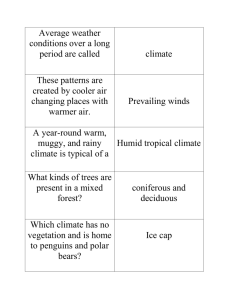
Supporting Information Notes S1–S3 Notes S1 The discovery of photoperiodism by G. Klebs (1914). Klebs G. 1914. Über das Treiben der einheimischen Bäume, speziell der Buche. Abhandl. Heidelberger Akad. Wiss. (Math. Nat. Kl.), No. 3, 1–112. This unusually long, pioneering paper is hard to read - even for a German-speaker. It was not quoted in the scientific English literature until 20 yr after its publication. Even then citations did not convey Klebs’ experimental evidence for the control of bud dormancy by the seasonality of insolation. The following detailed summary is apparently the first one published in English. We therefore give page numbers of the original paper for the observations summarized below. Experiments done in autumn 1913 All potted experimental beech (Fagus sylvatica) plants were dormant in September, but had some green leaves when they were moved to the “light chamber”, a dark room illuminated continuously by electric light bulbs. Experiments with saplings and seedlings In Exp. 1 -4 dormant beech plants were placed into light chamber progressively later. Legend: Light chamber Greenhouse O O Dormant ♠ ♠ Flushing Degree of dormancy:↓ +14 d = bud break after 14 d in light chamber S O ↓ +14d Exp. 1 p.17-19 O O N D J F ↓ +32d M ♠ ♠ ♠ ♠O O O O O O O O O O O O ♠ ♠ ♠ ♠ Conclusion: Bud expansion is inhibited by short days in October - December S O N D J F M Exp. 2 ↓ +21d ↓ +28d p.37 O O O ♠ ♠ ♠ ♠O O O O O O O ♠ ♠ ♠ ♠ Conclusion: Repeated transfers between autumnal short days and continuous light result in alternating periods of rest and shoot growth. Borchert et al., Insolation -1- Supporting Information S O N Exp. 3 p.20-25 Fig. S1a O O O O D ↓ +33 d J F M ♠♠♠♠♠♠♠♠♠♠♠♠♠♠♠♠♠♠ 15 new leaves expand during continous growth over four months. Conclusion: Saplings have the potential for continuos growth. S O N D Exp. 4 p.37-38 Fig. S1b O O O O O O J F M ↓ +39d ↓ +32d ♠♠ ♠♠ ↓ +42d ♠♠ Conclusions: Under continuous light shoots may alternate between bud break and rest. Exp. 1 - 4 Conclusions: With declinind day length in autumn, the number of long days required to induce bud break increases from 14d to 39d, i.e ., saplings are progressively more dormant. Notes S1 figure Prolonged shoot growth induced by exposure of dormant Fagus sylvatica (beech) to continuous light (a) Expansion of 15 leaves during 4-month-long continuous shoot growth of a sapling (see Exp. 3). (b) Expansion of three consecutive shoots. I, II, III – bases of three consecutive flushes (see Exp. 4; figures from Klebs, 1914). Borchert et al., Insolation -2- Supporting Information Experiments with isolated stem segments standing in water Exp. 5 (p. 54): Effect of day length: Oct. 5: cuttings placed into LC or GH > Oct. 20: bud break in LC, but not in GH. Conclusion: In autumn continuous light induces bud break, but natural short days do not. Exp. 6 (p.55-56): Effect of light intensity. Bud lengths observed at increasing distance from the light bulb: 40 cm > 8 mm bud; 80 cm > 5 mm; 160 cm > 2 mm; 200 cm > 0 mm. Conclusion: Bud break is a function of light intensity. Exp. 7 (p.57-59) Effect of duration of light periods. Light bulbs located 25 cm over glass vessels with cuttings. During dark periods vessels are covered by a dark box. Treatment Dec-Jan Feb. 1 T6 6h --- T12 12 h ----- T15 15 h T18 T24 18 h 24 h Feb. 15 Mar. 5 --- to T24 ----- +++ +++ +++ Buds: --- dormant +++ open Results: 1. In December/January T18 (18 h light-period) does not cause bud break, but by early February dormancy has been broken. 2. T15 causes bud break by early March, but not in February. 3. T12 does not cause bud break by early February, but transfer from T12 to T24 breaks dormancy within 2 wk. Conclusion: Light quantity determines bud break. General conclusions Photoperiodic control of temperate trees under natural conditions Borchert et al., Insolation -3- Supporting Information 1. Beech buds become progressively more dormant in autumn as light quantity (intensity x duration) and temperature decline. 2. In February increasing light quantity induces changes in dormant beech buds which enable bud break as soon as temperature permits. Shoot growth patterns of tropical and temperate trees Experimental manipulation of beech reveals three general shoot growth patterns of trees: 1. Tropical trees: Continuous shoot elongation (see above Notes S1 figure (a)) or 2. periodic shoot elongation without formation of resting buds protected by bud scales. 3. Temperate trees: Periodic shoot growth accompanied by the formation of resting buds (see above Notes S1 figure (b)). 4. Notes S2 Methods In our earlier studies of tree phenology in Central American semi-deciduous forests we observed trees biweekly for at least 2 yr. Such studies do not exist for equatorial forests. We therefore analyzed information on tree phenology near the equator obtained from the following sources: the Missouri Botanical Garden Herbarium (Borchert, 1996; Rivera & Borchert, 2001); our short-term field observations made in Colombia and Uganda; long-term field observations recorded earlier in the equatorial Amazon rainforest (Alencar et al., 1979; Alencar 1990; Gautier & Spichiger, 1986); synchronous flushing and flowering of canopy trees observed from low-flying aircraft; satellite-based remote sensing records of periodic greening in equatorial rainforests in South America and Africa (Huete et al., 2006; Mynemi et al., 2007; Guan et al., 2013). We analyzed herbarium collections of 420 tree species represented in the Tropicos data base of the Missouri Botanical Garden by more than 100 specimens. The majority of Borchert et al., Insolation -4- Supporting Information species are large evergreen trees, for which flowering specimens are quite rare. All wide-ranging species represented by a good number of flowering specimens are relatively small, easy-to-observe trees. They are semi-deciduous at higher latitudes, where most flowering specimens were collected within 2–3 months during the dry season (Fig. 1a–c). Near the equator flowering specimens are found throughout the year, i.e., the flowering periodicity is not as distinct as at higher latitudes and we show only values exceeding 5% of the total flowering herbarium collections of a species. For most semi-deciduous species the number of flowering specimens collected near the equator is too small to reveal distinct flowering periods. The relatively high number of herbarium specimens collected south of the equator in Ecuador and Bolivia, and the scarcity of collections from Colombia and Peru reflect differences in collecting activity, not species distribution. Notes S3 Molecular controls of tree development Synchronous bud break and flowering of the tropical tree species analyzed here are therefore likely to be manifestations of FT- control. Bud break induced by increasing insolation in February/March has been observed in heated glasshouses at temperate latitudes (Klebs, 1914), in temperate tree species growing in tropical Mexico (Borchert et al., 2005) and in evergreen trees at all tropical latitudes (Fig. 3b). FT is also likely to control flushing during both periods of increasing insolation near the equator (Fig. 1c) and the latitudinal variation of flushing time in Guazuma (Fig. 2a,b). Lastly, both the FTcontrolled onset of dormancy in Populus and autumn-flowering of many tropical trees are induced by declining photoperiod and start progressively later with declining latitude (Böhlenius et al., 2006; Calle et al., 2010). Thus, the molecular mechanisms of photoperiodic control of tree development are likely to be the same in temperate and tropical trees. Borchert et al., Insolation -5- Supporting Information References Alencar JC, Almeida RA, Fernandes NP. 1979. Fenologia de espécies florestais em floresta tropical úmida de terra firma na Amazônia Central. Acta Amazonica 9: 163–198. Alencar JC. 1990. Interpretação fenológica de espécies lenhosas de Campina na reserve biológica de Campina do INPA ao norte de Manaus. Acta Amazonica 20: 145– 183. Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss S, Nilson O. 2006. CO/FT module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043. Borchert R. 1996. Phenology and and flowering periodicity of neotropical dry forest species: evidence from herbarium collections. J. Trop. Ecol. 12: 65–80. Borchert R, Rivera G. 2001. Photoperiodic control of seasonal development and dormancy in tropical stem-succulent trees. Tree Physiol. 21: 213–221. Borchert R, Robertson K, Schwartz MD, Williams-Linera K. 2005. Phenology of temperate trees in tropical climates. Int. J. Biometeorol. 50: 57–65. Calle Z, Schlumberger BO, Piedrahita L, Leftin A, Hammer SA, Tye A, Borchert R. 2010. Seasonal variation in daily insolation induces bud break and flowering in the tropics. Trees 24: 865–877. Gautier L, Spichiger R. 1986. Ritmos de reproducción en el estrato arbóreo del Arboretum Jenato Herrera. Candollea 41: 193–207. Guan K, Wolf A, Medvigy D, Caylor KK, Pan M, Wood EF. 2013. Seasonality of coupling/decoupling of canopy functions and structure on African tropical forests and their environmental controls. Ecosphere 4: art35. Huete AR, Didan K, Shimabukuro YE, Ratana P, Saleska SR, Hutyra LR, Yang W, Nemani RR, Myneni R. 2006. Amazon green-up with sunlight in dry season. Geophys. Res. Lett. 33: L06405. Klebs G. 1914. Über das Treiben der einheimischen Bäume, speziell der Buche. Abhandl. Heidelberger Akad. Wiss. (Math. Nat. Kl.), No. 3, 1–112. Borchert et al., Insolation -6- Supporting Information Myneni RB, Yang W, Nemani RR, Huete AR, Dickinson RE, Knyazikhin Y, Didan K, Fu R, Juarez RIN et al. 2007. Large seasonal swings in leaf area of Amazon rainforests. PNAS 104: 4820–4823. Rivera G, Borchert R. 2001. Induction of flowering in tropical trees by a 30-min reduction in photoperiod: evidence from field observations and herbarium specimens. Tree Physiology 21: 201–212. Borchert et al., Insolation -7- Supporting Information