determination of chloride contents in floor layers of bathrooms – riyadh
advertisement

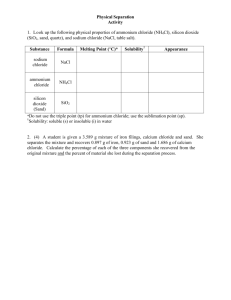
DETERMINATION OF CHLORIDE CONTENTS IN FLOOR LAYERS OF BATHROOMS – RIYADH By: Mahmoud M. Idris College of Architecture and Planning -King Saud University, Riyadh, Saudi Arabia KEYWORDS: Floor Layers, GI Pipes, Corrosion, Chloride Ions, Dextrin Suspension, end point. ABSTRACT Corrosion attack occurs as a result of chemical interaction of a metal with its surrounding. The prime cause of corrosion of metals embedded in the building fabric (i.e., GI water pipes, steel reinforcement…etc.) is the presence of high level of chloride contents in the building materials and water. Results of chemical analysis have shown that the level of chloride contents in the various floor layers of bathroom and tap water are very high, compared with the recommended limits by different codes and organizations. Eventually, this creates a favorable environment for the corrosion of water pipes embedded within floor layers of bathrooms and kitchens. Solutions for such a problem have to be worked out in this study. والمسبب الرئيس لتآكل معادن.تحدث هجمات التآكل في المعدن نتيجة لتفاعلها مع الوسط المحيط بها :الملخص الخ) هو وجود نسبة عالية من...العناصر المدفونة في جسم المبنى (مثل أنابيب المياه الصرف وقضبان التسليح كما،ًالكلوريد في المواد المستخدمة في طبقات أرضيات الحمامات والمياه مقارنة بالنسب المسموح بها عالميا دلت بذلك نتائج التحليل الكيميائي الذي أجري لبعض العينات المجمعة من طبقات األرضيات والماء لعدد من وبالتالي تكون طبقات المواد المستخدمة في أرضيات الحمامات باإلضافة إلى المياه المستخدمة.الحمامات لذا كان من الضروري البحث في.لألغراض المختلفة قد ساهمت في خلق البيئة المناسبة لحدوث عملية التآكل .هذا الموضوع بغرض الوصول لمعالجة هذه المشكلة INTRODUCTION Agents with whom metal comes in contact may define corrosion as the destructive chemical attack of metal. In fact, the destruction occurs as a result of the interaction of metal with its surrounding environment (Addleson and Rice, 1991). Under certain conditions related to water, temperature and composition, the contact of natural water with inorganic materials–metallic or non-metallic–may lead to formation of mineral deposits at the solid-liquid surface (Halleux, 1982). Also, if the materials are wetted, metals embedded inside components of building may corrode and may be brought into aqueous contact with other metals or materials which may lead to electrolytic attack (Ransaw 1980). The agencies responsible for the attack vary from material to material and from one location to another. According to (Addleson and Rice 1991), whether the attack is likely to occur depends on the chemical properties of the material, its exposure to dampness and the nature and composition of the particular agency within the material. In practice, different metals may be in contact with moisture so as to behave like small battery (galvanic cell). However, current and thus corrosion may also be set up in single metals when one part becomes anode and another the cathode. In both cases, the moisture involved may contain air or other dissolved chemical substances, which conduct electricity. Variations in physical conditions, which may give rise to the setting up of currents, include differences in temperature, stray currents, and water flow. (Buenfeld et al., 1998) reported that chloride ion is the main cause of Corrosion of steel reinforcements in concrete. Chloride may penetrate through concrete cover from the external environment or be present in the original mixmaterials. They explained that the process in the propagation of corrosion is the migration of chloride ions from the cathode to the anode in the electric field generated by the corrosion cell. Chloride ions are highly aggressive and capable of breaking down, or preventing the formation of protecting film. Chloride is usually found to be contaminated with the original mix ingredients (aggregates and water) (Matta, 1993). In addition, laden soil and water may come in contact with concrete allowing penetration of salts into even high quality concrete. On the other hand, moisture plays an important role in promoting corrosion especially in areas like bathrooms and kitchens, where the major source of water found in buildings. Inspection of a number of defective bathrooms under maintenance in the King Saud University (KSU) staff flats, have shown serious deterioration of water pipes buried within the bathrooms floors (Fig. 1). Galvanized steel pipes ( 1525mm) are used for both cold and hot water supply. UPVC pipes of sizes 50 and 110mm are used for waste water and soil disposal respectively. Both water supply and disposal pipes are buried in the sand filling below the terrazzo floor finish. Samples of sand filling concrete screed, terrazzo tiles from 13 bathrooms together with samples of water from 4 different locations were collected. The materials of these layers and water samples are tested to determine the level of chloride contents. Fig. (1): Corroded Pipes Concentration limits of chloride-ions vary widely from place to place. Many national codes and publications from various countries give different limits of chloride-ions by weight of cement. According to (Matta 1992), ACI committees differ somewhat on their recommendations for maximum chloride concentrations. While the ACI committee 201 recommends a limit of 0.10% watersoluble chloride ions by weight of cement, the ACI 318-83 requires a maximum of 0.15%, and the ACI 222 allows a maximum of 0.20%. He added that other national codes and publications give more liberal limits, as the Norwegian code N5 3474, Chloride Ion Limits: which allows 0.6% and the CIRIA publication No. 31 that recommends 0.3% chloride ions by weight of cement. These limits largely based on studies carried out in foreign countries. These limits, beside the (CIRIA 1984) limits are used widely in the Gulf region. (Table 1) summarizes the limits for total chloride and sulphates in concrete as recommended by (CIRIA 1984). Table (1): Recommended Limits for Total Chloride and Sulphates Content in Concrete Type of Concrete Reinforced concrete made with Portland cement containing less than 4% C3A (e.g. sulphate resisting Portland cement) Reinforced concrete made with Portland cement containing 4% or more C3A (OPC and ASTM type I & II usually contain more than 4% C3A) Un-reinforced concrete Max. Chloride (C1) Contents (% by Weight of Cement) Max. Sulphate (SO3) Content (% by Weight of Cement) All cases 4.0 including the sulphate ions in the cement 0.15 0.30 0.60 Source: CIRIA Guide to concrete construction in the Gulf Region Publication # 31 (ref. # 7) According to CIRIA, experience in the Gulf region (and elsewhere) has shown that the maximum chloride contents which is unlikely to cause corrosion at a serious rate in uncarbonated concrete is about 0.5% C1 by weight of cement. Where concrete has partly carbonated, the amount of chloride needed to promote corrosion is even lower. It is therefore extremely important that the concrete cover to the reinforcement should be impermeable. Conditions in Riyadh: According to (Hanson 1992), corrosion problems in Saudi Arabia of embedded steel frequently occurred at or near ground level. It appears that chloride-laden soils or water come in contact with the concrete, or chloride-laden water may be applied to concrete, allowing penetration of chlorides into even highquality concrete. Capillary action may also draw the chlorides up the near-surface region of the concrete. Intermittent wetting and drying of moisture condensation on concrete floor–as in case of bathrooms or kitchens–also supports corrosion process. Water and Aggregate: The water in the Gulf region has usually high chloride ion concentration, about 30000 ppm (Hanson 1992). The main source of water in Riyadh comes from desalination plants in Jubail 400 Km to the East of Riyadh. The water is fed into the city network via cast iron pipes, and then to the buildings network and appliances via galvanized steel pipes and chrome-plated steel or brass/copper fittings (Fig. 2). Both cold and hot water pipes are buried within the building fabric (walls and floors) (Figs. 3 and 4). Because of the permeable nature of the building materials, i.e. sand filling concrete...etc., which may also contain some oxygen and the chemical nature of the infilling material, corrosion, is likely to start by the electrochemical process. This may take place, as the infilling material gets wet from leaking pipes and water coming from above and through the finishing material creating a favorable environment for corrosion. Fig. (2): Salt from Water Leak through Pipe Joint The level of the ground water in Riyadh is rising at an average rate of 1m annually. The total amount of excess water penetrating into the soil in Riyadh is estimated about 64000m3 per day (Al-Shaikh et. al., 1992). The soil and ground already contaminated with high levels of sulphates (between 284 – 1733 mg/L) and chlorides (between 221 – 1841 mg/L) (Al-Towegri and Al-Tabba, 1992). Hence, any additional water will dissolve more of the salts and raise the risk of corrosion of embedded pipes. FLOOR FINISH SAND FILLING DISPOSAL PIPE SUPPLY PIPES FLOOR SLAB FLOOR FINISH SAND FILLING DISPOSAL PIPE SUPPLY PIPES FLOOR SLAB Fig. (3): Typical Details of Floor Layers in Bathrooms According to (Al-Idi and Al Mehthl 1995), concrete deterioration in Saudi Arabia is mainly due to the presence of chlorides and sulphates. Samples obtained from batch plants in the Eastern and Central Provinces of Saudi Arabia were tested. The results have shown that the inability of the local aggregates to meet the limits established by the industry standards. The aggregates in the Central Province (where Riyadh is situated) have 70% acid soluble chlorides (C1) and 55% acid soluble sulphates (SO4), compared to 3% and 40% British Standard (BS 1881) limits, respectively, (Table 2). Fig. (4): Pipe Buried Within Floor Layers of Bathrooms Table (2): Local Aggregate Property Eastern Province Central Province I.S. Limits Absorption 100 % 100 % 2.5 (BS 5337) Abrasion 100 % 100 % 50 (ASTM C33) Acid Soluble chlorides 95 % 70 % 0.03 (BS 1881) Acid soluble chlorides Sulphates(SO 3 ) 55 % 55 % 0.4 (BS 1881) 90 % (20 mm) 37 % (10 mm) 100 % (20 mm) 74 % (10 mm) 18 (ASTM C33) Soundness Source: Al-Idi and Al-Mehthl, Application of available technologies for production of Durable concrete, 4th Saudi Engineering Conference, 1995. (ref. # 3). The climate of Riyadh is characterized by the extreme variations of temperature (between 43oC in summer and 8oC in winter), humidity, and radiation (MDA, 1990). Coupled with this, the high levels of carbon monoxide (10-20 ppm), sulpher dioxide (15-30 ppm) and hydrogen sulphide (0.02ppm) present in the atmosphere (Al-Awat and Ba Sahby 1985). In addition, the sulphate and nitrates were found in the dust-fall in Riyadh at low levels of 0.3 and 0.09 tons/Km2 per month respectively. Lead particles were also found at higher levels between 4.0-9.0 µg/m3, which exceed that of 1.5 µg/m3 in the EEC (El Shobokshy et al., 1990). Eventually, these high levels of concentration of gases in the atmosphere coupled with extreme variations of temperatures will speed the rate of deterioration of building materials. Physical Environment: MATERIALS AND METHODS Samples of building materials from different floor layers i.e., sand filling, concrete screed and terrazzo tiles, together with pieces of GI water pipes from 13 defective bathrooms under maintenance in King Saud University (KSU) staff flats were collected. Each sample is kept in polythene bag, firmly tied and labeled. The labels indicate the number of building and flat floor level, and the name of the layer. The samples are ground into a very fine powder to ensure homog-eneity and to have a true representation of the sample. Samples of tap water from four different flats were also prepared for chemical analysis. A list of the required chemical tests is prepared and submitted to the KSU Department of Chemistry. Chemical Analysis Procedure: The chemical analysis for the samples is carried out following the procedure described below. Materials of the Layers: Weigh out in weighing dish 5gm of each samples; then transfer to 250ml Erlenmeyer flasks, and dissolve in 50ml distilled water, add 10ml 1% dextrin suspension, close tightly and shake well for one hour before using. Add 10 drops of dichlorofluorescien indicator solution. The dextrin prevents excessive coagulation of the precipitate at the end point. This keeps a larger surface area for absorption of the indicator, which enhance the sharpness of the end point. Filter the solution in 250ml volumetric flask. The whole solution is filtered and the residue is washed with distilled water, filtered in the same flask, and diluted to the mark. Take 25 ml of the sample for the determination of chloride ions concentration in the sample materials. Titrate with standard silver nitrate (AgNO 3 ) solution to the end point. A blank containing distilled water, dextrin, and dichlorofluorescien were titrated with standard silver nitrate (Ag NO 3 ) solution for calibration. Fajans method is used for the determination of chloride as soluble chloride (Christian 1994 The chemical analysis is done in collaboration with the Department of Chemistry, College of Science, King Saud University). The principle is that: the sample is titrated with standard Ag NO 3 (silver nitrate) solution, using dischlorofluorescin adsorption indicator end point. The analytical procedure is based on the following reactions: Ag + C1 AgC1 Ag + SCN AgSCN (brick-red suspension) Where; Ag: Silver, Cl: Chloride, AgCl: Silver Chloride, SCN: Thiocynate, AgSCN: Silver Thiocynate If the color of the suspension (AgSCN) turns to brick-red this means that the solution has reached the end point. When finished, put all silver-containing solutions in a jar provided for this purpose. Then calculate and report the percent C1 for each portion titrated in the sample. Water Samples: The analytical procedure for the determination of chloride (Cl-) in tap water is based on the following reactions: Preparation of Samples for Chemical Analysis: Hg (SCN)2 + 2 Cl- → HgCl2 + 2SCN2SCN- + Fe3+ → Fe (SCN)+ Where; Hg: Mercury, HgCl: Mercuric chloride, Fe3: Iron 3 (Ferric), Fe SCN: Iron Thiocynate. The chloride contents of the water samples react with Hg (SCN-)2 liberating SCN-, which in turn forms with Fe (III) the red–colored complex ion (Fe SCN)+ 2 . This is measured spectrophotometrically at 480nm. RESULTS The results of the chemical analysis for the determination of chloride concentration in the samples of the various floor layers in 13 bathrooms and water samples are summarized in (Table 3 and 4). The results have shown very high concentration of chloride (ranging from 814 ppm to 822 ppm) in the sand filling, where GI water and drainage pipes are buried. On the other hand, the chloride contents are very high in the screed layer and the terrazzo tiles (ranging from 1195 ppm to 1912 ppm). These values approach the maximum allowable limit recommended by the various codes; 200 ppm (0.02%) of ACI 318-83, 600 ppm (0.06%) of the Norwegian code N5 3474 and the CIRIA limit of 300 ppm (0.03%). Table (3): Chloride Concentration in Samples of Various Floors Layers of Bathroom Chloride Concentration (ppm) in Samples No. Label Location Sand Filling Concrete Screed Terrazzo Tiles 1. B22F10 2nd Floor 820 1412 1220 2. B38F09 2nd Floor 822 1459 1441 3. B27F09 2nd Floor 817 1445 1315 4. B42F03 Ground Floor 819 1382 1278 st 5. B05F06 1 Floor 821 1395 1312 6. B37F01 Ground Floor 822 1425 1399 7. B19F02 Ground Floor 821 1225 1195 8. B38F03 Ground Floor 821 1422 1325 9. B39F04 Ground Floor 814 1435 1412 10. B29F03 Ground Floor 815 1912 1622 11. B21F14 3rd Floor 822 1432 1325 12. B21F04 Ground Floor 817 1412 1325 13. B42F09 2nd Floor 814 1416 1389 The histograms in (Fig. 5) represent the variations in the levels of chloride contents in the different layers forming bathrooms floors. Results of the water analysis have shown higher values of chloride contents in the range of 360 ppm to 365 ppm which exceed the limits of 350 ppm and recommended by the (WHO1984) and adopted by The Saudi Arabian Standards Organization (SASO 1984). This means that water inside pipes is the main cause of corrosion to the material of the pipe than the external layers of the floor. Eventually, this may weaken pipes walls causing holes allowing for water leak. 2000 1500 Sand Filling Concrete Screed 1000 Terrazzo Tiles 500 B42F09 B21F04 B21F14 B29F03 B39F04 B38F03 B38F03 B19F02 B37F01 B05F06 B42F03 B27F09 B38F09 0 B22F10 Level of Chloride (C1) in ppm 2500 Number of Flats Fig. (5): Level of Chloride Contents in Various Floors Layers Table (4): Chloride (Cl-) Contents in Tap Water Item 1 2 3 4 Water Sample Sample No. 1 Sample No. 2 Sample No. 3 Sample No. 4 Chloride Content (ppm) 360 362 365 362 DISCUSSION Results of the chemical analysis of the raw materials of the samples (before grinding), gave a very high chloride contents in the range of ⋍ 6250 to ⋍ 7000 ppm. This indicates that these results are not typical of a representative sample. Samples are then ground into fine powder to ensure homogeneity of the materials and consistency of values. Even though the average values of chloride contents in the various layers are still high compared with the maximum levels recommended by many codes and organizations. The only justification for such high values could be resulting of chloride accumulation over a period of time, i.e., water tends to evaporate leaving the chloride ions to deposit within the materials of the layers. The source of chloride is usually found to be contaminated in the original mix ingredient (Matta, 1993). Aggregates in the Central Province, where Riyadh is situated, have 70% acid soluble chlorides (Al Idi and Al Mehthl, 1995). In addition to high chloride contents in the water and the soil. A geotechnical test prepared by Saudi Labs Ltd., (1992) for the staff flats, indicates high water salinity (TDS = 3885 ppm), with high chloride contents (865 ppm) and high sulphate contents (1580 ppm); and higher concentrations of sulphates (1774-2185 ppm) and moderate concentration of chloride (113-149 ppm) in the soil. All cement products are characterized by their comparatively large initial drying shrinkage, which makes them move widely (Addlesonands and Rice, 1991), creating cracks. In addition to their permeable nature, the concrete layers (screed and terrazzo tiles) allow chlorides to penetrate through them from external environment and raise the level of chloride already exists in the material. More-over, chloride ions may transport in concrete by diffusion due to concentration gradient or by water flow due to difference of pressure (capillarity) or absorption (Bunfeld et. al.,1998), and draw chloride up the surface of the material. Moisture plays an important role in promoting corrosion, and in most cases, moisture passing through concrete contains dissolved salts including chlorides depositing them within the floor layers. This is aggravated by the high rate of evaporation in Riyadh, and because of the high fluctuation in the annual rates of relative humidity (between 7% to 71%, Sharif 1975) and the daily variation between the early morning high rate and lower rates at mid-day. Also, the flats are left empty of occupants and dry of water during the summer vacation (June–August), after a long period of intensive use of water (from May to the end of September). According to (Ransaw 1980), water has high heat of vapourization and it is therefore slow to evaporate. Hence, in the bathrooms where plenty of water is used, it is possible that the excess water would penetrate through the porous materials of the floor finish. CONCLUSIONS Results from the chemical analysis have shown the presence of high levels of chloride contents in the different floor layers of bathrooms. Since chloride is the major agent causing corrosion of steel, then GI water pipes and other steel elements buried within any of these layers will be subjected to serious attacks of corrosion. Corrosion of embedded water pipes may take place internally (drinking water contains high contents of chloride) as well as externally where the pipes are surrounded by material (filling or concrete) with high chloride contents. Water leaking from these pipes may penetrate through the R.C. floor slabs leading to corrosion of steel reinforcement and GI pipes. Also causing damage to furniture, equipment and the interior decorations of upper spaces as well as causing damage to foundations at ground level. Solutions for such a problem may be in the following: 1-Using pipes made of materials other than steel-not affected by chloride. 2-Coat the GI pipes with protecting material against chloride (i.e., epoxy). 3-Using filling material, which is not affected by chloride. 4-Avoid, if possible, burying GI water pipes within building structure. REFERENCES 1- Al-Shaikh, Al-Refeai T. and Zaalouk A. (1992). Building Deterioration in Riyadh City- Case Study Proceeding of the Building Deterioration in the Arab World and Method of Repair Symposium, King Saud University, Riyadh, Kingdom of Saudi Arabia, PP: [400-412]. 2-Al Awat M., and Ba Sahby A. (1985). Pollution and Environment Safety, King Saud Univesity Libraries, Riyadh, (Arabic). 3-Al-Idi S. H. and Al-Mehthl M. H. (1995). Application of Available Technolo-gies for Production of Durable Concrete, the 4th Saudi Engineering Conference, Jeddah, Kingdom of Saudi Arabia. Vol. 2, PP: [241-51]. 4-Al Towegeri A. and Al-Tabba M. (1992). Effect of Water Table Level on Buildings in Riyadh. Proceeding of the Building Deterioration in the Arab World and Method of Repair Symposium, King Saud University, Riyadh, Kingdom of Saudi Arabia, PP: [367-88]. 5-Addleson L. and Rice C. (1991). Performance of Materials in Buildings, Butterworth, Oxford. 6-Buenfeld N. R., Glass G. K., Hassanein A. M., and Zhang J. (1998). Chloride transport in Concrete subjected to electric field, J. of Materials in Civil Engineering. Vol. 10, (4) PP: [220-27]. 7- CIRIA (1984). The CIRIA Guide to Concrete Construction in the Gulf Region, Special Publication # 31. 8- Christian G. D. (1994). Analytical Chemistry. John Wiley and Son, 5th Edition, New York. 9-El Shobokshy M. S., Tamarah S. A. and Hussien F. M. (1990). Invaluable Particulate and Meteorological Characteristics of the City of Riyadh, Saudi Arabia, Atmospheric Environment, 24B, PP: [261-65]. 10- Halleux G. I. D. (1982). New basic concepts in the evaluation of the Aggressiveness of natural waters, in "Bahtnagar V. M., Building Materials", The Construction Press, Lancaster. 11- Hanson J. M. (1992). Extending the Life of Concrete Structures, Proceed-ing of the Building Deterioration in the Arab World and Method of Repair Symposium, King Saud University, Riyadh, Kingdom of Saudi Arabia, PP: [557-75]. 12- Matta Z. G. (1992). Chlorides and Corrosion in the Arabian Gulf. Environment. Concrete International, Vol. 14, (5) PP: [47-48]. 13- Matta Z.G. (1993). More Deterioration of Reinforced Concrete in the Arabian Gulf, Concrete International, Vol. 15, (11) PP: [50-51]. 14- MDA (1990). Riyadh Climatological Normals (1961-1990), Ministry of Defense and Aviation, Riyadh, Kingdom of Saudi Arabia. 15- Ransow W. H. (1980). Building Failure, E and F.N. Spon, London. 16- Sharif A. S. (1975). The City of Riyadh, King Abdulaziz Foundation, Riyadh, Kingdom of Saudi Arabia (in Arabic). 17- SASO (1984). Saudi Arabian Standards Organization, Riyadh, Kingdom of Saudi Arabia. 18- Saudi Labs Ltd. (1992). Factual Report for Building No. 40 at KSU, Faculty Housing Riyadh. 19- WHO (1984). The World Health Organization Guidelines.