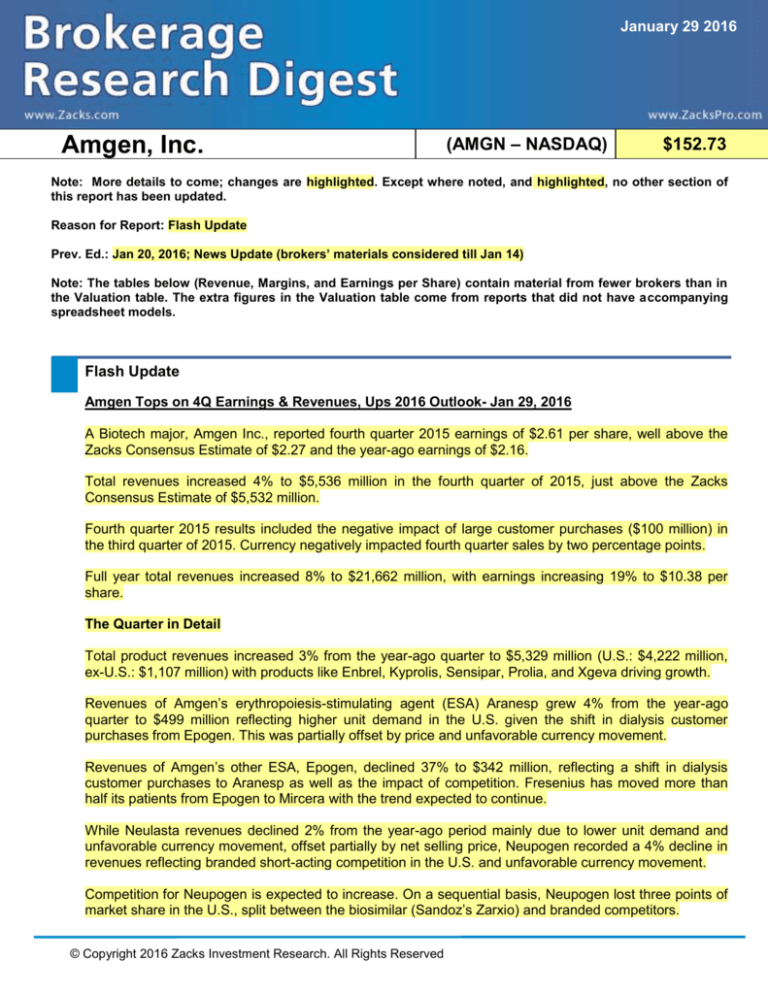
January 29 2016
Amgen, Inc.
(AMGN – NASDAQ)
$152.73
Note: More details to come; changes are highlighted. Except where noted, and highlighted, no other section of
this report has been updated.
Reason for Report: Flash Update
Prev. Ed.: Jan 20, 2016; News Update (brokers’ materials considered till Jan 14)
Note: The tables below (Revenue, Margins, and Earnings per Share) contain material from fewer brokers than in
the Valuation table. The extra figures in the Valuation table come from reports that did not have accompanying
spreadsheet models.
Flash Update
Amgen Tops on 4Q Earnings & Revenues, Ups 2016 Outlook- Jan 29, 2016
A Biotech major, Amgen Inc., reported fourth quarter 2015 earnings of $2.61 per share, well above the
Zacks Consensus Estimate of $2.27 and the year-ago earnings of $2.16.
Total revenues increased 4% to $5,536 million in the fourth quarter of 2015, just above the Zacks
Consensus Estimate of $5,532 million.
Fourth quarter 2015 results included the negative impact of large customer purchases ($100 million) in
the third quarter of 2015. Currency negatively impacted fourth quarter sales by two percentage points.
Full year total revenues increased 8% to $21,662 million, with earnings increasing 19% to $10.38 per
share.
The Quarter in Detail
Total product revenues increased 3% from the year-ago quarter to $5,329 million (U.S.: $4,222 million,
ex-U.S.: $1,107 million) with products like Enbrel, Kyprolis, Sensipar, Prolia, and Xgeva driving growth.
Revenues of Amgen’s erythropoiesis-stimulating agent (ESA) Aranesp grew 4% from the year-ago
quarter to $499 million reflecting higher unit demand in the U.S. given the shift in dialysis customer
purchases from Epogen. This was partially offset by price and unfavorable currency movement.
Revenues of Amgen’s other ESA, Epogen, declined 37% to $342 million, reflecting a shift in dialysis
customer purchases to Aranesp as well as the impact of competition. Fresenius has moved more than
half its patients from Epogen to Mircera with the trend expected to continue.
While Neulasta revenues declined 2% from the year-ago period mainly due to lower unit demand and
unfavorable currency movement, offset partially by net selling price, Neupogen recorded a 4% decline in
revenues reflecting branded short-acting competition in the U.S. and unfavorable currency movement.
Competition for Neupogen is expected to increase. On a sequential basis, Neupogen lost three points of
market share in the U.S., split between the biosimilar (Sandoz’s Zarxio) and branded competitors.
© Copyright 2016 Zacks Investment Research. All Rights Reserved
Meanwhile, Neulasta Onpro kit (on-body injector) is performing well, accounting for one-fourth of the
Neulasta business in the U.S. Amgen said that it does not expect biosimilar competition for Neulasta in
the U.S. until the end of 2016 assuming 180-day notice after approval.
Enbrel delivered revenues of $1,441 million, up 8% from the year-ago quarter, benefiting from net selling
price, offset partially by the impact from inventory changes and competition. Amgen reported a 2%
sequential decline in dermatology market share (22%) while rheumatology market share remained at
28%.
Prolia revenues came in at $380 million, up 21% from the year-ago quarter due to higher demand.
Meanwhile, Xgeva delivered revenues of $356 million, up 10% from the year-ago quarter due to higher
demand. Amgen intends to provide data on Xgeva from the event driven phase III study for the
prevention of skeletal-related events in patients with multiple myeloma in the fourth quarter.
Sensipar/Mimpara revenues increased 21% from the year-ago quarter to $384 million due to higher
demand and price. Vectibix revenues came in at $135 million during the quarter, up 2% from the yearago quarter reflecting higher unit demand, offset partially by unfavorable currency movement.
Kyprolis posted sales of $148 million, up 8% sequentially and 63% from the year-ago period reflecting
higher demand. Unit growth was driven by increased share and duration of treatment. The U.S. approval
in relapsed or second-line multiple myeloma has expanded the patient population significantly and should
boost sales further.
Amgen also provided an update on the launch of its PCSK9 inhibitor, Repatha. The company is yet to
break out sales of this product. However, Amgen said that although more than 80% of commercial lives
currently have access to Repatha, strict payer utilization management criteria are limiting the uptake.
Payer utilization management criteria are likely to remain fairly narrow until the outcomes data is
available (expected in the second half of 2016). Meanwhile, reimbursement discussions in individual
countries in the EU are ongoing.
While R&D expenses declined 9.5% from the year-ago period, SG&A spend grew 3.4%.
Ups 2016 Guidance
Amgen upped its 2016 outlook to reflect an improved revenue outlook due to revised timing assumptions
for biosimilar competition and the permanent extension of R&D tax credit. The company does not expect
Neulasta or Epogen biosimilars in the U.S. until the end of 2016 at the earliest, assuming potential
competitors provide 180-day notice between approval and launch.
The company now expects total revenues of $22.0 billion - $22.5 billion and earnings of $10.60 - $11.00
per share. Earlier, the company had guided towards total revenues of $21.7 billion - $22.3 billion and
earnings of $10.35 - $10.75 per share.
Amgen purchased shares worth $184 million during the reported quarter and intends to buy back shares
worth $2 billion - $3 billion in 2016.
Details, other news update and broker comments will be provided in the next edition.
Executive Summary
Amgen Inc. is the world’s largest biotechnology company. Its product portfolio includes Epogen for
anemia in kidney dialysis patients; Aranesp for anemia in the renal and oncology settings; Neupogen and
Neulasta for treating neutropenia; and Enbrel for inflammatory conditions. Other drugs include Nplate for
immune thrombocytopenic purpura (ITP), Vectibix for the treatment of colorectal cancer and
Zacks Investment Research
Page 2
www.zackspro.com
Prolia/Xgeva, an antibody targeting receptor activator of nuclear factor kappa-B ligand (RANKL) for postmenopausal osteoporosis (PMO) and bone metastases from solid tumors. In Oct 2013, Amgen acquired
biopharma company, Onyx and added products like Kyprolis (multiple myeloma), Stivarga (oncology) and
Nexavar (oncology) to its portfolio.
Of the 14 firms covering the stock, 8 firms (57.1%) gave neutral ratings and 6 firms (42.9%) gave positive
ratings. Notably, none of the firms were negative on the stock.
Neutral or equivalent outlook (8/14 firms): The firms are concerned about the stability of the
company’s key franchises. Additionally, they are concerned about the company’s dependence on a
handful of key products for a major part of its revenues. However, the firms believe the company has
interesting candidates in its pipeline and are particularly impressed by the biosimilars pipeline. The firms
are encouraged by the company’s strategic initiatives as well and believe the company is fairly priced at
current levels.
Positive or equivalent outlook (6/14 firms): The bullish firms are impressed by the performance of new
drugs like Sensipar. Some firms believe that the company’s future growth depends on Xgeva, Repatha
and Prolia and they see a lot of potential in these products. Further, Amgen’s pipeline is promising and
enthusiasm regarding romosozumab will keep increasing. Moreover, the company has a strong balance
sheet, which should provide significant strategic flexibility. Most of the bullish firms are pleased with the
company’s performance. The firms believe that investors have already factored in the impact of the loss
of patent exclusivity of the company’s key biologic products in the coming quarters. The firms are also
optimistic about Amgen’s biosimilars pipeline. They believe that the company’s maturing pipeline and
dividend policy will attract more interest.
Jan 20, 2016
Oct 8, 2015
Overview
Thousand Oaks, CA-based Amgen Inc. is a biotechnology company, engaged in the discovery,
development, manufacture and marketing of human therapeutics based on advances in cellular and
molecular biology. Amgen is a leading biotechnology company given the plethora of multi-billion dollar
products, including Epogen for anemia in kidney dialysis patients; Aranesp for anemia in the renal and
oncology settings; Neupogen and Neulasta for treating chemotherapy-induced neutropenia and Enbrel
for inflammatory conditions. The company also markets Vectibix for the treatment of colorectal cancer.
Amgen is focused on driving growth of Prolia, which has been approved for PMO and Xgeva, which is
approved for the treatment of bone metastases in advanced cancer. With the acquisition of biopharma
company, Onyx, Amgen added products like Kyprolis, Stivarga and Nexavar to its portfolio.
Amgen’s website is www.amgen.com.
The firms identified the following factors for evaluating the investment merits of Amgen:
Key Positive Arguments
New drugs like Sensipar are performing well. The
firms are also optimistic about the company’s
pipeline.
The clinical results of Prolia/Xgeva so far are positive
and most firms expect the drug to be the next growth
driver.
The company has undertaken initiatives like staff
reduction, rationalization of manufacturing facilities,
and outsourcing of non-core business functions to
help control costs. This in turn will lead to strong
earnings growth.
Zacks Investment Research
Key Negative Arguments
Amgen’s anemia franchise faces continued risks from
regulators, clinicians, payers and competitors, which
could result in stock volatility.
The company is highly dependent on Prolia/Xgeva for its
future growth. Any delay in gaining approval for
additional indications, disappointing results from
ongoing phase III programs, or safety concerns post
launch would be a setback for the stock.
Neupogen has started facing biosimilar competition in
the U.S. with the launch of Sandoz’s Zarxio. Enbrel and
Neulasta, too, could start facing biosimilar competition
soon.
Page 3
www.zackspro.com
Several firms are positive on the Onyx acquisition as
they expect it to improve the company’s growth
prospects.
Note: Amgen’s fiscal references coincide with the calendar year.
Jan 20, 2016
Long-Term Growth
Amgen possesses a strong and diversified pipeline of drugs that will boost the company’s long-term
growth potential. The majority of these are meant for the oncology and immunology markets.
Management has also been active in both in-licensing and out-licensing products.
Amgen’s strong cash position in the current economic environment allows the company to make strategic
acquisitions and, therefore, improve its long-term growth by purchasing undervalued products or
companies. Furthermore, it allows Amgen to use its cash reserves to return money to shareholders by
repurchasing shares opportunistically. Amgen is also using its cash for the payment of dividends. The
company plans to return an average of about 60% of the adjusted net income to its shareholders through
2018. The company’s focus will remain on dividend growth. In fact, it has increased its 1Q16 dividend by
27%.
Amgen has been pursuing acquisitions to expand its presence and strengthen its portfolio. A few firms
are positive on the Micromet acquisition as it leverages Amgen's experience in oncology and biologics in
addition to strengthening its product portfolio with Blincyto. This acquisition provided Amgen with the
novel BiTE (Bispecific T cell Engager) platform.
Amgen is working on expanding its operations in emerging markets and Japan. Amgen expects to
generate more than $2 billion in sales from new and emerging markets by 2018. Important target markets
include China and Japan, with Russia, Brazil and Turkey being other priority markets. The acquisition of
Mustafa Nevzat, a privately held Turkish pharmaceutical company, has significantly expanded Amgen's
presence in Turkey and the surrounding areas.
Amgen acquired Onyx in early Oct 2013 and strengthened its presence in the oncology market. Apart
from driving revenues, the acquisition has also boosted Amgen’s oncology pipeline. Most firms are
positive on this deal and are also pleased by the company’s commitment to growing dividends.
As far as the pipeline is concerned, pivotal data on several late-stage pipeline candidates are expected in
2016.
The company is also investing in biosimilars and has set up a small unit, which should contribute to
growth from 2017. The company has nine biosimilar candidates in its portfolio representing huge
commercial opportunity. Amgen expects to launch its first biosimilar in 2017 followed by four others
through 2019. Amgen also has a partnership with Allergan for biosimilars and estimates the biosimilars
opportunity could bring in sales of more than $3 billion annually.
Meanwhile, competitive pressure for most of the products in Amgen’s portfolio is expected to increase.
Biosimilar competition for some products remains a threat. Generic Zometa is also an issue for Xgeva.
Amgen remains on track with its restructuring plan which will see the company cutting 3,500–4,000
positions. Amgen expects to generate total annual savings of up to $1.5 billion and expects to improve its
adjusted operating margins by 52% to 54% by 2018.
Zacks Investment Research
Page 4
www.zackspro.com
Jan 20, 2016
Target Price/Valuation
Rating Distribution
Positive
Neutral
Negative
Avg. Target Price
High
Low
No. of Analysts with Target
price/Total
42.9%↑
57.1%↓
0.0%
$183.00
$200.00
$157.00
8/14
Risks to target price include earlier-than-expected entry of biosimilars in the U.S., pipeline and regulatory
setbacks.
Recent Events
Imlygic Gains European Approval – Dec 17
Amgen announced that the European Commission has approved the use of Imlygic (talimogene
laherparepvec) for the treatment of adults with unresectable melanoma that is regionally or distantly
metastatic (stage IIIB, IIIC and IVM1a), with no bone, brain, lung or other visceral disease.
This makes Imlygic the first oncolytic immunotherapy to be approved in Europe.
Hikes 1Q16 Dividend – Dec 15
Amgen declared a 27% increase in its quarterly dividend. The company announced a dividend of $1.00
per share for the first quarter of 2016, payable on Mar 8, 2016, to shareholders of record as of Feb 16,
2016. This represents an increase from $0.79 per share paid in each of the previous four quarters.
This latest hike takes the company’s yearly dividend to $4.00 per share, reflecting a forward yield of
nearly 2.5% based on the raised dividend and the stock’s last closing price of $162.62 (Dec 15, 2015).
Moreover, the hike is in accordance with the company’s dividend guidance for 2016 provided during the
3Q15 earnings call.
Regains Rights to Prolia, Xgeva & Vectibix from Glaxo – Dec 14
Amgen announced that it has entered into a definitive agreement with Glaxo. Under this agreement, the
former will regain all remaining rights to its bone drugs, Prolia and Xgeva, as well as cancer drug Vectibix
in 48 countries including Asia, South America, Europe, Australia and other regions over the world from
the latter. The deal includes countries that Amgen perceives as key markets for expansion, such as
Brazil, China, Colombia, Hong Kong, Israel, Singapore, South Korea, Taiwan and Thailand.
Glaxo had owned select regional rights to Prolia and Xgeva since 2009 and to Vectibix since 2010. It had
generated about $111 million in combined sales in 2014 by licensing the drugs from Amgen.
Per the deal, Amgen will make milestone payments (amount undisclosed) to Glaxo upon signing and
later, on successful transition of the products to itself. Post the switch, Amgen will book all sales for the
drugs. While majority of the markets will be shifted back to Amgen over a 12-month period, Glaxo will
Zacks Investment Research
Page 5
www.zackspro.com
continue to hold the license for as well as sell and distribute the products for an interim transition period
that will vary according to the countries.
Amgen expects the deal to be accretive to its adjusted earnings in 2017.
The latest deal with Glaxo is in line with Amgen’s strategy to expand its presence in international markets
that have significant commercial potential. It would further provide the company with a platform to
introduce new treatments targeting important therapeutic areas such as oncology and bone health.
New Phase II Data on Blincyto Presented – Dec 7
Amgen announced that new data from three phase II studies support the efficacy and safety of Blincyto
(blinatumomab) in adults with acute lymphoblastic leukemia (ALL). Data were presented at the annual
meeting of the American Society of Hematology. In a phase II confirmatory multicenter single-arm study
(BLAST), adults with B-cell precursor ALL with minimal residual disease (MRD) who received Blincyto
monotherapy demonstrated clinically meaningful relapse-free survival.
Other presentations showed Blincyto's potential in a high-risk sub-population of patients with relapsed or
refractory Philadelphia chromosome-positive (Ph+) B-precursor ALL and confirmed the drug’s efficacy in
a subset of patients with relapsed or refractory Ph- ALL after an allogeneic hematopoietic stem cell
transplantation, who usually have poor outcomes with current therapies.
Seeks Kyprolis Label Expansion – Dec 5
Amgen announced that results from a pivotal head-to-head phase III ENDEAVOR (RandomizEd, OpeN
Label, Phase 3 Study of Carfilzomib Plus DExamethAsone Vs Bortezomib Plus DexamethasOne in
Patients With Relapsed Multiple Myeloma) study on Kyprolis were published in The Lancet Oncology.
The study comparing Kyprolis in combination with low-dose dexamethasone versus Velcade
(bortezomib) and low-dose dexamethasone was conducted in patients with relapsed multiple myeloma.
The primary endpoint of progression-free survival (PFS) was met in patients on Kyprolis plus
dexamethasone living twice as long without their disease worsening and demonstrating superiority over
Velcade plus dexamethasone (18.7 months versus 9.4 months, respectively). Overall survival data is yet
to mature and continues to be monitored.
Amgen also announced the presentation of new key data evaluating Kyprolis-based regimens in patients
with relapsed multiple myeloma. Data showed that Kyprolis in combination with dexamethasone
significantly extended disease progression compared to Velcade plus dexamethasone across a range of
difficult-to-treat populations, particularly those with high risk and previously treated disease. Data were
presented at the annual meeting of the American Society of Hematology.
In a separate press release, the company announced the submission of a regulatory application to the
European Medicines Agency (EMA) seeking approval for Kyprolis in combination with dexamethasone for
the treatment of multiple myeloma patients who have received at least one prior therapy.
Inks Cancer Immunotherapy Deals with Merck – Dec 4
Amgen announced a couple of cancer immunotherapy collaborations with Merck. The first deal will
evaluate the safety and efficacy of Amgen’s Blincyto (CD19 bispecific T cell engager) in combination with
Merck’s Keytruda (anti-PD-1 therapy) in an open-label, multicenter, randomized phase Ib/III study in
patients with diffuse large B-cell lymphoma.
Meanwhile, the second deal will assess the safety and efficacy of Amgen’s AMG 820 (anti-colonystimulating factor 1 receptor antibody) in combination with Keytruda in an open-label phase I/II study in
Zacks Investment Research
Page 6
www.zackspro.com
patients with select advanced solid tumors including non-small cell lung, colorectal and pancreatic
cancers.
Seeks European Approval for First Biosimilar of Humira – Dec 4
Amgen announced the submission of a marketing authorization application to the EMA for ABP 501, a
biosimilar candidate to AbbVie’s best-selling drug, Humira (adalimumab). According to Amgen, this is the
first Humira biosimilar application submitted to the EMA. ABP 501 is Amgen's first biosimilar to be
submitted for approval in the EU.
Seeks FDA Approval for First Biosimilar of Humira – Nov 25
Amgen announced the submission of a biologics license application (BLA) seeking FDA approval for ABP
501, a biosimilar version of AbbVie’s best-selling drug, Humira. The application is Amgen’s first BLA
submitted under the 351(k) biosimilar pathway. The company believes that it is the first to file a biosimilar
application for Humira.
Revenue
Amgen reported total revenues of $5.72 billion in 3Q15, up 14% y/y, surpassing the expectations of
several firms. Products like Enbrel, Kyprolis, Sensipar, Prolia and Xgeva drove sales. The Zacks Digest
average total revenues in 3Q15 were in line with the company’s report.
Total product sales increased 14% y/y to $5.516 billion (U.S.: $4,425 million, ex-U.S.: $1,091 million) in
3Q15. Products like Enbrel, Kyprolis, Sensipar, Prolia, Neulasta and Xgeva drove growth. Price, low
inventory levels in the prior year period and higher unit demand drove growth. Unfavorable currency
movement had a 2 percentage point negative impact on top-line growth.
2015 Outlook: Amgen raised its revenue guidance and now expects total revenues of $21.4 billion $21.6 billion (old guidance: $21.1 billion - $21.4 billion).
2016 Outlook: Amgen also provided its preliminary outlook for 2016. The company expects revenues of
$21.7 billion - $22.3 billion. Negative currency movement is expected to impact revenues by about 1
percentage point.
Revenue ($
in million)
Total
Revenue
3Q14A
2014A
1Q15A
2Q15A
3Q15A
4Q15E
2015E
2016E
2017E
$5,031.0
$20,063.0
$5,033.0
$5,370.0
$5,723.0
$5,510.7↓
$21,638.4↓
$21,888.1↓
$22,729.3↓
Digest High
$5,031.0
$20,063.4
$5,033.0
$5,370.0
$5,723.0
$5,549.0
$21,675.0
$22,049.0
$22,743.6↓
Digest Low
$5,030.7
$20,062.0
$5,032.5
$5,370.0
$5,723.0
$5,480.2↓
$21,606.2↓
$21,753.0
$22,715.0
Specific Products
ESA (erythropoiesis stimulating agent) franchise - Aranesp and Epogen
Aranesp
Indication: Aranesp is indicated for the treatment of anemia both in supportive cancer care and
nephrology for patients on dialysis and those not on dialysis.
Mechanism: Aranesp is an erythropoietic protein that works by stimulating the bone marrow to produce
more red blood cells.
Zacks Investment Research
Page 7
www.zackspro.com
Product Life Cycle Status: Marketed
Safety Issues: The language in the label was modified to included changes to the Boxed Warning,
provides new information for the treatment of patients with chronic kidney disease (CKD) who are on
dialysis as well as those not on dialysis. The FDA approved a Risk Evaluation and Mitigation Strategy
(REMS) for ESAs, including Aranesp, Epogen and Procrit in Feb 2010. The REMS program includes a
“Dear Doctor” letter and a medication guide explaining the risks and benefits of ESAs, which must be
provided to all patients.
Sales: Amgen reported worldwide Aranesp sales of $493 million in 3Q15, up 4% y/y reflecting higher unit
demand, including a shift in dialysis customer purchases from Epogen. This was partially offset by price
and unfavorable currency movement. The Zacks Digest average revenues in 3Q15 were in line with the
company’s report.
Competitors: Johnson & Johnson’s Procrit and Roche’s Mircera. Aranesp also faces competition in
Europe from biosimilars marketed by companies like Pfizer (Hospira) and Novartis (Sandoz).
$ in million
Aranesp Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$1,930.0
$1,939.2↑
$1,873.9↑
$1,811.8
$1,679.9
-
Epogen
Indication: Anemia in CKD patients on dialysis
Mechanism: Epogen is an erythropoietic protein that works by stimulating the bone marrow to produce
more red blood cells. Epogen is an injectable drug.
Product Life Cycle Status: Marketed
Safety Issues: A boxed warning in the prescribing information notes that patients should be informed
about the increased risks of mortality, serious cardiovascular events, thromboembolic events and tumor
progression when used in off-label dose regimens or populations.
Sales: Amgen reported worldwide Epogen sales of $489 million in 3Q15, down 6% y/y reflecting a shift in
dialysis customer purchases to Aranesp as well as the impact of competition. This was partially offset by
price and favorable changes in inventory levels. Fresenius has moved more than half its patients from
Epogen to Mircera with the trend expected to continue. Apart from utilization at Fresenius, Epogen sales
in upcoming quarters will be impacted by potential switching to Aranesp and potential biosimilar
competition. The Zacks Digest average revenues in 3Q15 were in line with the company’s report.
Patents: Big players such as Pfizer and Novartis are already in the European market with generic
erythropoietin (EPO) products. The company lost patent protection for Epogen in May 2015. Pfizer is
currently seeking FDA approval for Retacrit, its biosimilar version of Epogen.
Competition: Roche’s Mircera
$ in million
Epogen Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$2,031.0
$1,962.4↑
$1,717.5↓
$1,557.8
$1,390.0
-
G-CSF
The company’s G-CSF franchise includes Neupogen and Neulasta. Neupogen is Amgen’s trademark
for filgrastim‚ which has been selected as the name for recombinant methionyl human granulocyte
colony-stimulating factor (r-metHuG-CSF).
Zacks Investment Research
Page 8
www.zackspro.com
Neupogen
Indication: Neutropenia or low white blood cell counts a side effect of chemotherapy
Mechanism: Neupogen stimulates the production of a type of white blood cells.
Product Life Cycle Status: Marketed
Safety Issues: Neupogen and Neulasta may be associated with increased risk of leukemia in breast
cancer patients who are initially treated with these agents in combination with chemo and/or radiation
therapy.
Sales: According to the company, 3Q15 sales of Neupogen decreased 5% y/y to $284 million reflecting
branded short-acting competition in the U.S. The Zacks Digest average revenues in 3Q15 were in line
with the company’s report.
Competitors: Biosimilars are starting to have a negative impact on Neupogen and Neulasta sales in the
EU. In the U.S., Teva launched Granix (tbo-filgrastim) in early Nov 2013. Granix is approved for the
reduction of the duration of severe neutropenia in certain types of cancer (non-myeloid malignancies)
patients who are receiving chemotherapy that affects the bone marrow.
Meanwhile, Teva has withdrawn its marketing application for its long-acting product candidate
(lipegfilgrastim) in the U.S.
Sandoz, Novartis’ generic arm, launched Zarxio, its biosimilar version of Neupogen in Sep 2015.
$ in million
Neupogen Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$1,159.0
$1,033.2↓
$911.2↓
$814.4
$767.0
-
Neulasta
Indication(s): Neutropenia or low white blood cell count, a side effect of chemotherapy. On-body injector
version approved in the U.S.
Product Life Cycle Status: Marketed
Sales: According to the company, 3Q15 sales of Neulasta grew 6% y/y to $1.267 billion driven by price
and favorable changes in inventory levels. Neulasta benefited from some abnormally large purchases by
some end customers in the U.S. this quarter, adding to the volume growth. Meanwhile, Neulasta Onpro
kit (on-body injector) is performing well and has already achieved 19% market share of the Neulasta
business. The Zacks Digest average revenues in 3Q15 were in line with the company’s report.
The company expects to face some Neulasta related challenges in 2016 due to the expiry of material
U.S. patents covering Neupogen.
Competitors: Sandoz has a biosimilar version of Neulasta under FDA review.
$ in million
Neulasta Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$4,596.0
$4,750.0↑
$4,377.0↑
$4,096.0
$3,816.0
-
Enbrel
Indication: Moderate-to-severe rheumatoid arthritis (RA), moderate-to-severely active polyarticular
juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis and moderate-to-severe plaque
psoriasis.
Zacks Investment Research
Page 9
www.zackspro.com
Product Life Cycle Status: Marketed
Safety Issues: The label includes warnings regarding the occurrence of lymphoma and other cancer
types in children and young adults using tumor necrosis factor (TNF) blockers like Enbrel.
Partners: Amgen has an agreement with Pfizer for the marketing of Enbrel in the U.S. and Canada. The
profit-sharing agreement converted into a royalty payment arrangement in Nov 2013.
Sales: Amgen reported Enbrel sales of $1.459 billion in 3Q15, up 30% y/y benefiting from price and low
inventory levels in the prior year period, partially offset by competition. Amgen reported that segment
growth remains strong in both rheumatology and dermatology which grew 25% and 28%, respectively.
However, the company recorded a 2% sequential decline in dermatology market share (24%) while
rheumatology market share remained at 28%. The Zacks Digest average revenues in 3Q15 were in line
with the company’s report.
Outlook: Enbrel sales are expected to reach $5 billion before the launch of Amgen's biosimilar
adalimumab.
Competitors: Johnson and Johnson’s Simponi and Stelara, UCB’s Cimzia, AbbVie’s Humira, BristolMyers’ Orencia and Roche’s Actemra. Sandoz has a biosimilar version of Enbrel under FDA review.
$ in million
Enbrel Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$4,688.0
$5,260.0↓
$5,322.5↓
$5,204.0
$5,211.0
-
Sensipar/Mimpara
Indication: Sensipar is indicated for the treatment of secondary hyperparathyroidism in patients with
CKD on dialysis.
Product Life Cycle Status: Marketed
Sales: Amgen reported worldwide Sensipar/Mimpara sales of $353 million in 3Q15, up 29% y/y due to
higher demand, low inventory levels in the prior year period and price. The Zacks Digest average
revenues in 3Q15 were in line with the company’s report.
Outlook: Sensipar sales are expected to reach $1.5 billion by 2018.
Patents/Generics: Amgen has filed a patent infringement lawsuit against Teva. On Jan 7, 2011, a U.S.
District Court in Delaware granted an injunction preventing Teva from launching generic versions of
Sensipar until the drug’s U.S. patents expire. The last patent expires in 2018.
$ in million
Sensipar Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$1,158.0
$1,375.0↓
$1,336.7↑
$1,395.2
$1,077.2
-
Vectibix (panitumumab)
Indication: Treatment of patients with wild-type KRAS (exon 2 in codons 12 or 13) metastatic colorectal
cancer (mCRC) as determined by an FDA-approved test for this use: as first-line therapy in combination
with FOLFOX; as monotherapy following disease progression after prior treatment with fluoropyrimidine-,
oxaliplatin-, and irinotecan-containing chemotherapy.
In the EU, Vectibix is indicated for the treatment of adult patients with wild-type RAS mCRC in first-line in
combination with FOLFOX and FOLFIRI; in second-line in combination with FOLFIRI for patients who
Zacks Investment Research
Page 10
www.zackspro.com
have received first-line fluoropyrimidine-based chemotherapy (excluding irinotecan) and as monotherapy
after failure of fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens.
Product Life Cycle Status: Marketed
Safety Issues: The label includes a warning regarding dermatologic toxicities.
Partners: Takeda (for Japan) and Zhejiang Beta Pharma Co., Ltd. (for China).
Sales: Amgen reported worldwide Vectibix sales of $132 million in 3Q15, down 4% y/y, reflecting
unfavorable currency movement that was partially offset by strong unit growth in the U.S. and EU. The
Zacks Digest average revenues in 3Q15 were in line with the company’s report.
Competitors: Lilly’s Erbitux
Recent Data: In Jun 2015, Amgen announced that Vectibix plus best supportive care (BSC)
demonstrated a statistically significant improvement in overall survival in patients with chemorefractory
wild-type KRAS (exon 2) mCRC compared to patients on BSC alone in a phase III study.
$ in million
Vectibix Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$505.0
$546.9↓
$613.7↓
$639.2
$675.9
-
Nplate
Indication: ITP, an autoimmune disease characterized by low platelet count.
Product Life Cycle Status: Marketed
Importance: Nplate, the first FDA-approved peptibody protein, works by raising and sustaining platelet
counts, thus representing a novel approach for the treatment of this chronic disease.
Regulatory Issues: Nplate’s U.S. prescribing information makes it clear that it is not indicated for
thrombocytopenia associated with myelodysplastic. In Dec 2011, the FDA modified the REMS to
eliminate the restricted distribution and reduce the burden on physicians. The FDA will no longer require
prescribers to complete clinical assessment forms as part of the REMS.
Sales: Amgen reported worldwide Nplate sales of $137 million in 3Q15, up 15% y/y. The Zacks Digest
average revenues in 3Q15 were in line with the company’s report.
Competitors: Novartis’ Promacta
$ in million
Nplate Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$469.0
$528.0
$572.5↓
$618.0
$660.0
-
Prolia (denosumab)
Indication: Approved in the U.S. for the treatment of PMO, treatment of bone loss in patients with
prostate or breast cancer undergoing hormone ablation therapy (approved in Sep 2011), and increasing
bone mass in men with osteoporosis at high risk of fracture (approved in Sep 2012). EU approval
includes the treatment of bone loss associated with hormone ablation in men with prostate cancer at an
increased risk of fractures.
Mechanism: RANKL antibody.
Product Life Cycle Status: Marketed
Zacks Investment Research
Page 11
www.zackspro.com
Importance: Most of the firms believe that Prolia has high potential and will become a major growth
driver, going forward. The firms, in general, believe that if Prolia is successfully marketed for both the
osteoporosis and cancer indications, the revenue potential could well exceed $2 billion, bringing
considerable growth to Amgen’s top and bottom line.
Sales: Amgen reported Prolia sales of $320 million in 3Q15, up 25% y/y driven by higher demand. The
response to a new DTC campaign, launched in 2014, remains strong and has resulted in an increase in
patient awareness and has resulted in unit share gains in the U.S. as well as Europe. The Zacks Digest
average revenues in 3Q15 were in line with the company’s report.
Competitors: Novartis’ Reclast, Merck’s Fosamax, Roche’s Boniva and Lilly’s Evista and Forteo.
Recent Data: Amgen presented phase III data on Prolia at ASCO in Jun 2015 demonstrating that Prolia
significantly reduced bone fractures in breast cancer patients receiving aromatase inhibitors.
$ in million
Prolia Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$1,030.0
$1,300.5↓
$1,420.5↑
$1,550.0
$1,712.0
-
Xgeva (denosumab)
Indication: Approved in the U.S. and EU for the prevention of SREs in patients with bone metastases
from solid tumors. However, it is not indicated for the prevention of SREs in patients with multiple
myeloma. Approved in the U.S. for the treatment of giant cell tumor of bone (GCTB), in adults and
skeletally mature adolescents, which cannot be treated with surgery.
Product Life Cycle Status: Marketed
Sales: Xgeva sales were $378 million in 3Q15, up 19% y/y reflecting higher demand. The Zacks Digest
average revenues in 3Q15 were in line with the company’s report. Xgeva benefited from some
abnormally large purchases by some end customers in the U.S. this quarter, adding to the volume
growth.
The company continues to work on driving Xgeva growth by focusing on its superior clinical profile, more
sales force focus, and direct-to-patient programs.
Competitors: Novartis’ Zometa and generic versions of Zometa
$ in million
Xgeva Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$1,221.0
$1,356.0↑
$1,528.0↓
$1,356.0
$1,528.0
-
Kyprolis (carfilzomib)
Indication: Treatment of multiple myeloma patients who have received at least two prior therapies,
including Velcade (bortezomib) and an immunomodulatory agent, and whose disease has progressed on
or within 60 days of completion of the last therapy. Approved by the FDA in Jul 2015 for use in
combination with Revlimid and dexamethasone (KRd) in multiple myeloma patients who have received
one to three prior lines of therapy.
KRd combination approved in the EU in Nov 2015 for the treatment of adults with multiple myeloma who
have received at least one prior therapy.
Stage of Development: Marketed
Zacks Investment Research
Page 12
www.zackspro.com
Regulatory Issues: Under priority review in the U.S. for use in combination with dexamethasone for the
treatment of patients who have received at least one prior therapy with a response expected by Jan 22,
2016. In addition, a regulatory application for the same indication has been filed in the EU.
Sales: The company reported Kyprolis sales of $137 million in 3Q15, up 15.1% sequentially and 46% y/y
reflecting higher demand. The company reported strong unit growth driven by increased share and
duration of therapy. The Zacks Digest average sales for 3Q15 were in line with the company’s report.
Amgen expects continued sales growth as new relapsed patients start and stay on therapy for a longer
duration.
Recent Data: In Dec 2015, Amgen announced that results from a pivotal head-to-head phase III
ENDEAVOR (RandomizEd, OpeN Label, Phase 3 Study of Carfilzomib Plus DExamethAsone Vs
Bortezomib Plus DexamethasOne in Patients With Relapsed Multiple Myeloma) study on Kyprolis were
published in The Lancet Oncology. The study comparing Kyprolis in combination with low-dose
dexamethasone versus Velcade (bortezomib) and low-dose dexamethasone was conducted in patients
with relapsed multiple myeloma. The primary endpoint of PFS was met in patients on Kyprolis plus
dexamethasone living twice as long without their disease worsening and demonstrating superiority over
Velcade plus dexamethasone (18.7 months versus 9.4 months, respectively). Overall survival data is yet
to mature and continues to be monitored.
Amgen also announced the presentation of new key data evaluating Kyprolis-based regimens in patients
with relapsed multiple myeloma. Data showed that Kyprolis in combination with dexamethasone
significantly extended disease progression compared to Velcade plus dexamethasone across a range of
difficult-to-treat populations, particularly those with high risk and previously treated disease. Data were
presented at the annual meeting of the American Society of Hematology.
Ongoing Studies: Amgen is evaluating a weekly dosing regimen of Kyprolis in a phase III study
(ARROW) in relapsed and refractory multiple myeloma patients. Data should be out by 2017.
Results from another head-to-head (CLARION) study with Velcade in newly diagnosed patients are
expected in 2017.
Competitors: Celgene’s Pomalyst
$ in million
Kyprolis Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$331.0
$507.0↓
$741.0↑
$1,170.0
$1,420.0
-
Stivarga (regorafenib)
Indication: mCRC in treatment-experienced patients and the treatment of metastatic and/or
unresectable gastrointestinal stromal tumors (GIST) in patients whose disease progressed despite prior
treatment.
Stage of Development: Marketed
Partners: Bayer
Sales: The Zacks Digest average Stivarga royalty revenues were $22.6 million in 3Q15.
$ in million
Stivarga Royalty
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$69.2
-
-
-
-
-
Nexavar (sorafenib)
Zacks Investment Research
Page 13
www.zackspro.com
Indication: Unresectable hepatocellular carcinoma or liver cancer and advanced renal cell carcinoma or
advanced kidney cancer, locally recurrent or metastatic, progressive, differentiated thyroid carcinoma
refractory to radioactive iodine therapy.
Product Life Cycle Status: Marketed
Partners: Bayer
Sales: The Zacks Digest average Nexavar royalty revenues were $95.7 million in 3Q15.
Competitors: Pfizer’s Sutent, Inlyta and Torisel, Roche’s Avastin, Novartis’ Afinitor and Votrient
$ in million
Nexavar Collaboration
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$333.7
Blincyto (blinatumomab)
Indication: Ph- relapsed or refractory B-cell precursor ALL
Product Life Cycle Status: Marketed
Sales: According to the Zacks Digest model, Blincyto delivered sales of $18.5 million in 3Q15.
Additional Studies: Blincyto is in a phase II study for the treatment of minimal residual disease
positive ALL and in a phase III study in adults suffering from relapsed, refractory ALL with results
expected in 2016.
Blincyto is also being developed for the treatment of non-Hodgkin's lymphoma (NHL) and has the
potential to be developed for other hematologic malignancies including pediatric relapsed/refractory ALL.
Blincyto became a part of Amgen’s pipeline following its Mar 2012 acquisition of biotech company,
Micromet.
Additional Data: In Dec 2015, Amgen announced that new data from three phase II studies support the
efficacy and safety of Blincyto in adults with ALL. Data were presented at the annual meeting of the
American Society of Hematology. In a phase II confirmatory multicenter single-arm study (BLAST), adults
with B-cell precursor ALL with MRD who received Blincyto monotherapy demonstrated clinically
meaningful relapse-free survival. Other presentations showed Blincyto's potential in a high-risk subpopulation of patients with relapsed or refractory Ph+ B-precursor ALL and confirmed the drug’s efficacy
in a subset of patients with relapsed or refractory Ph- ALL after an allogeneic hematopoietic stem cell
transplantation, who usually have poor outcomes with current therapies.
$ in million
Blincyto Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$1.8
$44.0↑
$114.5↓
$160.0
$185.0
-
Corlanor (EU trade name: Procoralan)
Indication: Reduction of the risk of hospitalization for worsening heart failure in patients with stable,
symptomatic chronic heart failure with left ventricular ejection fraction ≤35%, who are in sinus rhythm with
resting heart rate ≥70 beats per minute (and either are on maximally tolerated doses of beta blockers or
have a contraindication to beta blocker use.
Product Life Cycle Status: Marketed
Sales: According to the Zacks Digest model, Corlanor delivered sales of $1.4 million in 3Q15.
Zacks Investment Research
Page 14
www.zackspro.com
$ in million
Corlanor Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$0.0
-
-
-
-
-
Repatha (evolocumab/AMG-145)
Indication: Adjunct to diet and maximally tolerated statin therapy for the treatment of adults with
heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease, who
require additional lowering of LDL-C; and as an adjunct to diet and other LDL-lowering therapies for the
treatment of patients with homozygous familial hypercholesterolemia, who require additional lowering of
LDL-C.
Importance: Repatha is a fully human monoclonal antibody that inhibits PCSK9.
Product Life Cycle Status: Marketed
Partner: Astellas for Japan (approval expected in 1H16)
Sales: According to the Zacks Digest model, Repatha delivered sales of $0.7 million in 3Q15. The
company reported that the U.S. launch is off to a good start. With Express Scripts placing Repatha on its
formulary, Amgen continues to negotiate with other payers to expand access in the U.S. CVS has given it
exclusive coverage. However, the company noted that it expects payer’s utilization management criteria
to remain fairly narrow until the outcomes data is available (expected in 2H16). Reimbursement
discussions in individual countries in the EU are ongoing. Quite a few firms are optimistic about this
product and believe it has huge market potential.
Ongoing Studies: Results from a long-term phase III outcomes study (FOURIER) on Repatha and the
company’s vascular imaging phase III study on the candidate are expected in 2H16. Amgen is also
evaluating Repatha in a phase III study to determine its effects on atherosclerotic disease burden, as
measured by intravascular ultrasound in patients undergoing coronary catheterization.
Additional Formulation: A 420-mg monthly dose of Repatha as a single injection for administration is
currently under FDA review with a response expected by Jul 10, 2016. It is also under review in the EU.
Competitors: Sanofi/Regeneron’s Praluent. Potential competitors that could enter the market include
Pfizer’s bococizumab (phase III), Alnylam/The Medicines Co.'s ALN-PCSsc.
Legal Issues: In Oct 2014, Amgen filed a lawsuit against Sanofi and Regeneron for patent infringement.
These patents describe and claim monoclonal antibodies to PCSK9. Sanofi and Regeneron completed
phase III studies on alirocumab (a monoclonal antibody targeting PCSK9) and intend to pursue
regulatory approval to market alirocumab in the U.S. The trial date is set for Mar 7, 2016.
$ in million
Repatha Sales
2014A
2015E
2016E
2017E
2018E
Est. Growth (‘14-’17)
$0.0
$34.0↓
$283.5↓
$620.0
$1,050.0
$34.0
Imlygic (talimogene laherparepvec)
Indication: Local treatment of unresectable cutaneous, subcutaneous and nodal lesions in patients with
melanoma recurrent after initial surgery. Approved in the EU for the treatment of adults with unresectable
melanoma that is regionally or distantly metastatic (stage IIIB, IIIC and IVM1a), with no bone, brain, lung
or other visceral disease.
Product Life Cycle Status: Marketed;
Ongoing Studies: Amgen is studying Imlygic in combination with Yervoy in a phase II study with results
due in 2016. Amgen also has an agreement with Merck under which Imlygic is being evaluated in
Zacks Investment Research
Page 15
www.zackspro.com
combination with Merck’s anti-PD-1 immunotherapy, Keytruda, in a phase II study in patients with midto late-stage melanoma. Results from this study are expected in 2017. A global, randomized phase III
trial evaluating the combination in patients with regionally or distantly metastatic melanoma is being
initiated as well. Moreover, the combination will be evaluated in a phase I, open-label trial in patients with
recurrent or metastatic squamous cell carcinoma of the head and neck.
Imlygic will also be evaluated in combination with Roche’s anti-PDL1 candidate, atezolizumab (also
known as MPDL3280A), in a phase Ib study in patients with triple-negative breast cancer and colorectal
cancer with liver metastases.
Pipeline Candidates
Romosozumab (AMG 785): Romosozumab is a monoclonal antibody against sclerostin that is being
developed for osteoporosis. Amgen and partner UCB are conducting two phase III studies with
romosozumab for the treatment of PMO. While data from the placebo-controlled registrational study
should be out in 1Q16, data from the Fosamax(alendronate)-controlled-study are expected in 2017.
In Sep 2015, the companies announced positive top-line data from the phase III Forteo- (teriparatide)
controlled STRUCTURE (STudy evaluating effect of RomosozUmab Compared with Teriparatide in
postmenopaUsal women with osteoporosis at high risk for fracture pReviously treated with
bisphosphonatE therapy) study. The primary endpoint was achieved with romosozumab showing a
statistically significant difference in the percent change of total hip bone mineral density (measured by
DXA) through month 12.
Omecamtiv mecarbil: Omecamtiv mecarbil is a novel cardiac myosin activator being studied for the
treatment of heart failure. In Nov 2015, Amgen announced the presentation of data from the expansion
phase of COSMIC-HF (Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure),
a phase II study evaluating omecamtiv mecarbil in patients with chronic heart failure. The study met its
primary pharmacokinetic objective and showed statistically significant improvements in all pre-specified
secondary measures of cardiac function in the treatment group employing pharmacokinetic-based dose
titration.
In Sep 2013, Amgen had presented data on omecamtiv mecarbil at the European Society of Cardiology.
Amgen said that omecamtiv mecarbil failed to meet its primary endpoint in the randomized, double-blind,
placebo-controlled phase II ATOMIC-AHF (Acute Treatment with Omecamtiv Mecarbil to Increase
Contractility in Acute Heart Failure) study.
Although omecamtiv mecarbil missed the primary endpoint, other data points from the study were
encouraging especially the data related to the incidence of worsening heart failure in the placebo group.
Omecamtiv mecarbil is being developed in collaboration with Cytokinetics. Servier has European
commercialization rights to omecamtiv mecarbil.
Etelcalcetide: Amgen is currently seeking U.S. (FDA action date: Aug 24, 2016) and EU approval for
etelcalcetide for the treatment of secondary hyperparathyroidism (SHPT) in adult CKD patients on
dialysis. Etelcalcetide became a part of Amgen’s portfolio following its Jul 2012 acquisition of privately
held KAI Pharmaceuticals. With this acquisition, Amgen gained global rights (excluding Japan) to
etelcalcetide. SHPT is a common and serious complication for the CKD patient population.
In Feb 2015, Amgen announced results from a head-to-head phase III study comparing etelcalcetide to
Sensipar for the treatment of SHPT in patients with CKD receiving hemodialysis. The study met the
primary endpoint of non-inferiority to Sensipar.
ABP 501 (adalimumab): Amgen is developing ABP 501 as a biosimilar to AbbVie’s Humira which is
approved in a number of countries for the treatment of several inflammatory diseases including RA,
Zacks Investment Research
Page 16
www.zackspro.com
plaque psoriasis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis,
Crohn's disease and ulcerative colitis. In Nov 2015, Amgen presented detailed findings from a head-tohead phase III study comparing the safety, efficacy and immunogenicity of ABP 501 with Humira in
patients with moderate-to-severe RA. The study met its primary endpoint. Regulatory applications both in
the U.S. and EU have been filed.
AMG 334: AMG 334 is a fully human monoclonal antibody in phase III studies for the prevention of
migraine. In May 2015, Amgen announced results from a global phase II, double-blind, placebocontrolled study evaluating AMG 334’s efficacy and safety for the prevention of episodic migraine. The
primary endpoint of reducing monthly mean migraine days compared with placebo was achieved. Phase
IIb chronic migraine data should be out in 2H16.
Agreements, Collaborations and Acquisitions
In Dec 2015, Amgen announced a couple of cancer immunotherapy collaborations with Merck. The first
deal will evaluate the safety and efficacy of Amgen’s Blincyto (CD19 bispecific T cell engager) in
combination with Merck’s Keytruda (anti-PD-1 therapy) in an open-label, multicenter, randomized phase
Ib/III study in patients with diffuse large B-cell lymphoma.
Meanwhile, the second deal will assess the safety and efficacy of Amgen’s AMG 820 (anti-colonystimulating factor 1 receptor antibody) in combination with Keytruda in an open-label phase I/II study in
patients with select advanced solid tumors including non-small cell lung, colorectal and pancreatic
cancers.
In Dec 2015, Amgen announced that it has entered into a definitive agreement with Glaxo. Under this
agreement, the former will regain all remaining rights to its bone drugs, Prolia and Xgeva, as well as
cancer drug Vectibix in 48 countries including Asia, South America, Europe, Australia and other regions
over the world from the latter. The deal includes countries that Amgen perceives as key markets for
expansion, such as Brazil, China, Colombia, Hong Kong, Israel, Singapore, South Korea, Taiwan and
Thailand.
Glaxo had owned select regional rights to Prolia and Xgeva since 2009 and to Vectibix since 2010. It had
generated about $111 million in combined sales in 2014 by licensing the drugs from Amgen. Per the
deal, Amgen will make milestone payments (amount undisclosed) to Glaxo upon signing and later, on
successful transition of the products to itself. Post the switch, Amgen will book all sales for the drugs.
While majority of the markets will be shifted back to Amgen over a 12-month period, Glaxo will continue
to hold the license for as well as sell and distribute the products for an interim transition period that will
vary according to countries.Amgen expects the deal to be accretive to its adjusted earnings in 2017.
In Sep 2015, Amgen announced a research and license agreement with clinical-stage biotech company,
Xencor, for the development and commercialization of novel treatments targeting areas of cancer
immunotherapy and inflammation. The agreement focuses on bringing together Amgen's expertise in
target discovery and protein therapeutics and Xencor's XmAb bispecific technology platform. The
collaboration covers molecular engineering by Xencor and the preclinical development of bispecific
molecules for five programs proposed by Amgen. The deal also includes a preclinical bispecific T cell
engager program directed at CD38 and CD3 for multiple myeloma. Amgen will be solely responsible for
preclinical and clinical development and commercialization across the world. The deal could see Xencor
receive an upfront payment of $45 million and payments of up to $1.7 billion on the achievement of
certain clinical, regulatory and sales milestones for the six programs. Additionally, Xencor is entitled to
mid-to-high single-digit royalties for the candidates directed against Amgen's targets and high single to
low double-digit royalties for Xencor's CD38 bispecific T cell engager.
In Oct 2015, Amgen acquired Dezima Pharma, a privately held Dutch biotech company focused on
developing treatments for dyslipidemia. The acquisition adds Dezima's late-stage lead pipeline
candidate TA-8995 – an oral, once-daily CETP inhibitor – to Amgen’s cardiovascular portfolio. Amgen
Zacks Investment Research
Page 17
www.zackspro.com
paid $300 million in cash at closing and has agreed to make additional payments of up to $1.25 billion on
the achievement of certain development and sales milestones. Amgen will also pay low single-digit
royalties on net sales on exceeding a certain threshold. Dezima had licensed the rights to TA-8995 from
Mitsubishi Tanabe Pharma Corporation. Dezima will pay Mitsubishi part of the upfront, development and
sales milestone payments plus royalties on net sales if a certain threshold is reached. Moreover,
Mitsubishi will retain development and commercialization rights to TA-8995 in certain territories in Asia
including Japan.
In Sep 2015, Amgen announced a neuroscience collaboration with Novartis for Alzheimer's disease and
migraine. The worldwide co-commercialization and co-development pact will focus on both companies’
BACE (beta-site APP-cleaving enzyme-1) programs targeting Alzheimer's disease. While Novartis' BACE
inhibitor CNP520 (phase I/IIa) will be the lead molecule under the collaboration, the pre-clinical BACE
inhibitor programs at each company will be treated as potential follow-ons.
Amgen will make upfront and milestone payments as well as disproportional R&D costs for an agreedupon period following which cost and profit will be split equally. On the other hand, Novartis will get global
co-development and commercial rights to Amgen’s portfolio of experimental migraine candidates – AMG
334 (phase III) and AMG 301 (phase I) – outside the U.S., Canada and Japan. Moreover, Novartis
receives an option to commercialize an additional early-stage Amgen molecule in these territories.
Novartis will fund disproportional amounts of global R&D and pay double-digit royalties on sales in return
for these territorial rights.
Please refer to the Zacks Digest spreadsheet on AMGN for further details on revenue estimates.
Margins
The company reported research and development (R&D) expenses of $1.086 billion in 3Q15, up 10.8%
y/y. Selling, general and administrative (SG&A) expenses increased 17.4% y/y to $1.206 billion in
3Q15.
According to the Zacks Digest model, 3Q15 R&D and SG&A expenses were in line with the company’s
report.
Margins
Gross
Operating
Pre-Tax
Net
3Q14A
2014A
1Q15A
2Q15A
3Q15A
4Q15E
2015E
2016E
2017E
84.9%
84.8%
85.4%
85.3%
87.0%
85.2%↑
85.8%↑
84.9%
86.0%↓
45.0%
42.2%
48.7%
47.5%
46.9%
42.7%↑
46.4%
47.9%↑
50.4%
42.4%
39.2%
45.8%
46.0%
44.4%
39.8%↑
44.0%↑
45.2%
48.3%↓
35.2%
33.4%
38.0%
36.8%
36.4%
29.9%↓
35.2%↓
35.8%↑
39.1%↑
Outlook: 4Q15 expenses are slated to increase about $200 million from 3Q15 reflecting normal
spending patterns and investment in product launches.
Restructuring Initiative: During 2H14, Amgen initiated a restructuring plan and remains on track to cut
headcount by 3,500 to 4,000. Amgen plans to reduce its facilities footprint by approximately 23%. The
company expects to close its facilities in Washington State and Colorado and reduce the number of
buildings at its headquarters. In 2015, the company realized 16% reduction in facilities footprint.
Amgen expects to generate total annual savings of up to $1.5 billion (gross cost savings of $700 million
by the end of 2015 and $1.1billion by 2016 end) by 2018. In 2014, the company realized around $300
million of cost saving of its expected savings of $1.5 billion. The company expects incremental cost
savings of more than $400 million in 2015.
Amgen expects its adjusted operating margin to improve to 52%–54% by 2018.
Zacks Investment Research
Page 18
www.zackspro.com
Note: According to the Zacks Digest model, cost of goods sold (COGS) is expected to increase 0.6% in
2015 and 2.9% in 2016; SG&A expenses are expected to increase 4.6% in 2015 and decrease 7.7% in
2016; and R&D expenses are expected to decrease 2.7% in 2015 and 1.5% in 2016. In comparison,
revenues are expected to increase 7.9% in 2015 and 1.2% in 2016.
Please refer to the Zacks Digest spreadsheet on AMGN for further details on margins.
Earnings per Share
Amgen reported 3Q15 earnings per share of $2.72, 18.3% above the year-ago figure. Earnings were
above the expectations of several firms. The Zacks Digest average earnings per share in 3Q15 were in
line with the company’s report.
EPS
Digest High
Digest Low
Digest Average
3Q14A
2014A
1Q15A
2Q15A
3Q15A
4Q15E
2015E
2016E
2017E
$2.31
$8.72
$2.48
$2.57
$2.72
$2.25
$10.03
$10.60↑
$12.13↑
$2.30
$8.69
$2.48
$2.57
$2.72
$2.02↓
$9.80↓
$10.29
$11.75
$2.30
$8.70
$2.48
$2.57
$2.72
$2.17↓
$9.95↓
$10.44↑
$11.94↑
2015 Outlook: Amgen raised its 2015 earnings guidance range to $9.95–$10.10 per share (old
guidance: $9.55–$9.80 per share). Some firms have raised their earnings estimates.
Share Buyback: Amgen repurchased approximately $2 billion of shares in 2015.
2016 Outlook: Amgen also provided its preliminary outlook for 2016. The company expects earnings of
$10.35 - $10.75 per share. Negative currency movement is expected to impact EPS by 12 cents.
Amgen expects cumulative share repurchases worth about $4–$5 billion from Oct 2014 through 2016
end. In 2016, the company intends to buy back shares worth about $2–$3 billion.
The company expects to return about 60% of adjusted net income to its shareholders through 2018.
Please refer to the Zacks Digest spreadsheet on AMGN for further details on EPS.
Analyst
Poushali Bagchi
Last Updated
Arpita Dutt
Lopamudra
Bhattacharya
Copy Editor
Content Editor
Lead Analyst
Arpita Dutt
QCA
Arpita Dutt
Reason for Update
Flash Update
Zacks Investment Research
Page 19
www.zackspro.com