Prelab for Experiment #1: Extraction
advertisement

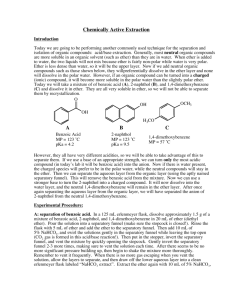
Prelab for Experiment #2: Extraction Your Name: Lab partner’s name: Name of TA: Lab Section: Title: Experiment #1: Extraction Purpose: In this lab, we will partition materials between various solvents, based upon their acid/base properties. Reagents Table: Name of Reagent Biphenyl Benzoic Acid p-Nitroaniline Methylene Chloride 3M HCl 3M NaOH 6M NaOH 6M HCl M.W. (g/mol) 154 122 138 85 M.P. (oC) 70 122 146 B.P. (oC) 39.8 Density (g/ml) 1.32 Hazards toxic Contact hazard poison Carcinogen, toxic Corrosive, Toxic Corrosive, Toxic Corrosive, Toxic Corrosive, Toxic Procedure: 1. Weigh out 3 grams of the chemical mixture. Dissolve the mixture in 40 ml of CH2Cl2 with gentle heating. After all solids have dissolved, pour the solution into a separatory funnel. 2. Rinse the initial flask (used to dissolve mixture) with 10 ml CH2Cl2 and pour this solution to the separatory funnel as well. 3. Add 15ml of 3M HCl and mix gently and vent. Allow the layers to separate and determine which one is the organic layer. Collect the top (aqueous layer) in a container labeled HCl extract. Place the lower organic layer into the separatory funnel and re-extract with another 15ml of 3M HCl. Collect the aqueous extraction from the second extraction in the same container that was labeled HCl extract. Keep this container (HCl extract) on ice. Once the HCl extract is cold, add 6M NaOH dropwise till a precipitate is formed and red litmus paper turns blue. Chill solid and vacuum filter solid followed by washing the crystals with chilled water. 4. Dry crystals and find the weight of p-nitroaniline. Do Not Recrystallize! 5. Place the lower organic layer once again in the separatory funnel. Add 15ml of 3M NaOH and mix gently and vent. Allow the layers to separate and collect the top (aqueous layer) in a container labeled NaOH extract. Place the lower organic layer back into the separatory funnel and re-extract with another 15ml of 3M NaOH. Collect the aqueous extraction from this extraction in the same container that was labeled NaOH extract. Keep this container (NaOH extract) on ice as well. Once the NaOH extract is cold, add 6M HCl dropwise till a precipitate is formed and blue litmus paper turns red. Chill solid and vacuum filter solid followed by washing the crystals with chilled water. 6. Dry crystals and find the weight of benzoic acid Take the remaining organic layer and add sodium sulfate until free floating salt is observed (~ 3 grams). Dry the organic layer for 5 minutes and filter the organic solution into a tared (preweighed) 100 ml round bottom flask. Remove the CH2Cl2 by rotary evaporator and find the weight of biphenyl in the flask. [This is the complete prelab for experiment 2. There are no prelab questions for this experiment, so if you were to show up for the lab with this level of detail, you would be good to go!] One other suggestion: you might want to use the columns in your lab notebook with one column as Procedures and the next column as Observations: Procedures 1. Weigh out 3 grams of the chemical mixture Observations 1. Weighed out 3.0148 grams of chemical mixture. 2. Dissolve the mixture in 40 ml of CH2Cl2 with gentle heating 2. Used 45 mL of CH2Cl2.