Mohammed S. Al-Saadi, Sherif AF Rostom* and Hassan M. Faid
advertisement

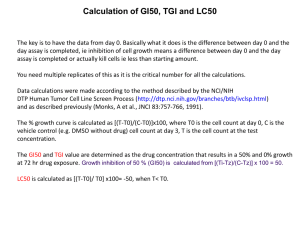
89 IN VITRO ANTITUMOR SCREENING OF SOME POLYSUBSTITUTED PYRAZOLE ANALOGS Mohammed S. Al-Saadi, Sherif A. F. Rostom* and Hassan M. Faid-Allah ك ربوكسضضيليأشيدضضيدشهيدرولويضضدشوم ض ش ض ش-3- بيضضزولو-يضضد1شهيدروكس ض ش-4-شكلوروفيني ض )ش-4( ش-1بعضضمشتق ض ش ش–شووكسض يي لوالي شووم ض شدضتشش قضييده ش4,2,1ش–ش زوي لواليض شوش4,2,1ششتثض ش3تج وع شإحالميةشتخ لفضةشفض شوم و ض ش وإجزوءشوم يضي شوم تضد شمنقض ا شوم ثض شفض شإي ض اشع ضوشومعديضدشتض شومخاليض شومسضز عيةشفض شوم عاضدشومضو ن شملسضز ش وذمأشف شوحدةشومغزبلةشومخ صةشب ض يو شومخاليض شومسضز عيةشوم ع ليضةشوم وجاضةشمل ضزقدششودضدشوجضدشفض شومسض ي ش ض ش شدضضدشيراضضز شف عليضضةشتضض يةشوودضضعةشوم ضضدخشملخاليض ش3-1وألوميضضةشم ليض شومخليضضةشفض شوم عاضضدشي شمالمضضةشتض شوم زكتض شهض ش ومسز عيةش دشتج شوود شتض ش وضو شومخاليض شومتقضزيةشوم خ لفضةشوذمضأشفض ش سض شتناض مشوهضت شوم زكتض ش ض شو ي رهض ش بوودوةشوم عادشومو ن شمت وثشومسز شمي شإجزوءشغزبلةش أكيديةشعليا شي صدشتنا ش يي شإتك عي شوم ج ربشوم ع ليضةش وم ضض يةشملسضز دششو ضنشتن دقضةشتفصضضلةشألفضض شع ض بشوم جض ربشوم ع ليضةشمل ضضأميزشوم ضض يشملخاليض شومسضز عيةشماضضت ش وم زكت ض شومنقضضوةشوم ض ش ض شوم صضضو شعليا ض شت ض شومتزو وكضضوال شومثالمضضةشوم ع دتضضةشومخ صضضةشب م عاضضدشومضضو ن شألب ض ثش شم ض ش ضضدوينا شف ض شهضضت شوم مضضةشودضضدشيراضضز شوم زكت ض شومفع مضضةشومثالمضضةشعنضضدشوم زكي ض شوألود ض شوم ثضضت شملن ضضوش ومسضضز د ش1>2>ش3 شت شيرجةشف عليةشت ق باةش زيتشششLD50 شو زكي شيود شت ينشTGIتس وي ش ثتي شع وشكل شGI50 ش ش Certain 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazole-3-carboxylic acid hydrazide derivatives, carrying various substituents at position 3- such as 1,2,4-triazolyl and 1,2,4-oxadiazolyl moieties have been previously synthesized and preliminarily evaluated for their antitumor activity at the National Cancer Institute’s (NCI) in vitro disease-oriented antitumor screen unit. In the primary 60 cell line assay, three compounds (1-3) revealed potential broad spectrum antitumor activity against a wide range of different human cell lines of nine tumor subpanels. These compounds were further selected by the NCI for carrying out confirmatory screening procedures in order to assess their exact in vitro antitumor potentialities. A detailed discussion of the best in vitro antitumor results of these active analogs obtained from the three consecutive NCI procedures is reported in this paper. The active three compounds at the GI50 level exhibited TGI and LC50 levels with nearly the same order of activity; 3> 2> 1. Key words: 4-Hydroxy-1H-pyrazoles, 1,2,4-Triazole, 1,2,4-Oxadiazole, Antitumor screening Introduction Research and development of new pyrazoles for cancer chemotherapy have been one of a major focus in anticancer drug design. In this respect, the discovery of the natural antibiotic pyrazofurin; 4hydroxy -3 β-D-ribofuranosyl -1H-pyrazole-5-carboxamide; having a potent antitumor efficacy in some Division of Medicinal Chemistry, Faculty of Medicine, King Abdul-Aziz University, P.O. Box 80205, Jeddah 21589, Saudi Arabia. *To whom correspondence should be addressed. Saudi Pharmaceutical Journal, Vol. 13, No. 2-3 April-July 2005 cancer cases (1) lead to intensive investigations of numerous pyrazoles carrying different functionalities for their anticancer (2-4), antiviral (5-7) as well as antimicrobial (8-10) potentials. During our ongoing studies aimed at the discovery of new heterocycles endowed with antitumor activity (11-13), we have presented the synthesis and the primary anticancer evaluation of several new 1-(4-chlorophenyl)-4hydroxy-1H-pyrazole-3-carboxylic acid hydrazide derivatives (13), according to the protocol of the National Cancer Institute’s (NCI) in vitro diseaseoriented antitumor screen unit. We have also emphasized the role of incorporating substituents at 90 AL-SAADI ET AL position 3- of the pyrazole ring such as triazolyl and oxadiazolyl moieties on the antitumor potency, where they showed an interesting activity often associated with high or moderate selectivity for certain human tumor cell lines. As a consequence, three of these compounds (1-3) were further selected by the NCI’s biological committee for subsequent confirmatory screening procedures in order to assess their exact antitumor potentialities. These tests include the repetition of the testing in the primary screen, referring to the Biological Evaluation Committee and a final review by the Biological Evaluation Committee. In the current NCI anticancer screen, each candidate is tested over a broad concentration range against every cell line in the panel (14-16). Active compounds are selected for further testing based on several criteria: disease-type specificity in the in vitro assay, unique structure, potency and demonstration of a unique pattern of cellular cytotoxicity or cytostasis, indicating a unique mechanism of action or intracellular target (17). Secondary in vitro studies to optimize the exposure time to an agent and to define its potential mechanism of action are useful for determining the necessity of the subsequent in vivo studies (17). In this paper, the best data obtained from the previously-mentioned three NCI in vitro screening procedures with a discussion of the antitumor activities of compounds (1-3) are presented. Materials and Methods Source of Compounds: The 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazole3-carboxylic acid hydrazide derivatives investigated in the present study were previously synthesized and characterized (13). Their chemical names are: -5-(1-(4-Chlorophenyl)-4-hydroxy-1H-pyrazol-3yl)-4-cyclohexyl-1,2,4-triazolin-3-thione (1) -5-(1-(4-Chlorophenyl)-4-hydroxy-1H-pyrazol-3yl)-1,3,4-oxadiazol-2-one (2) -5-(1-(4-Chlorophenyl)-4-hydroxy-1H-pyrazol-3yl)-2-phenyl-1,3,4-oxadiazole (3) In vitro Antitumor Testing and Data Analysis: Compounds 1-3 were subjected to the NCI in vitro disease-oriented human cells screening panel assay to screen their antitumor activities (14-16). About 60 cell lines of nine tumor subpanels, including leukemia, non-small cell lung, colon, CNS, melanoma, ovarian, renal, prostate and breast Saudi Pharmaceutical Journal, Vol. 13, No. 2-3 April-July 2005 cancer cell lines, were incubated with five concentrations (0.01-100 μM) for each compound and were used to create log concentration - % growth inhibition curves. Three response parameters (GI50, TGI, and LC50) were calculated for each cell line. The GI50 value (growth inhibitory activity) corresponds to the concentration of the compounds causing 50% decrease in net cell growth, the TGI value (cytostatic activity) is the concentration of the compounds resulting in total growth inhibition and the LC50 value (cytotoxic activity) is the concentration of the compounds causing net 50% loss of initial cells at the end of the incubation period (48h). Subpanel and full panel mean-graph midpoint values (MG-MID) for certain agents are the average of individual real and default GI50, TGI, or LC50 values of all cell lines in the subpanel or the full panel, respectively (14). The NCI antitumor drug discovery was designed to distinguish between broad spectrum antitumor compounds and tumor or subpanel-selective agents. Results and Discussion The selected three pyrazoles 1-3 revealed distinctive potential patterns of activity against some individual tumor cell lines (Table 1), as well as a broad spectrum of antitumor activity (Tables 2-4). Regarding the sensitivity against some individual cell lines, they proved to be very sensitive towards most of the tested subpanel tumor cell lines with significantly low GI50 values ranging between <0.01 – 59.8 μM. Compound 1 revealed sensitivity towards about 9 different cancer cell lines with GI 50 values lying in the nanomolar concentration range (GI50 values < 0.01 μM). Moreover, it showed a remarkable sensitivity against most of the tested subpanel tumor cell lines (GI 50 values <0.01 – 6.05 μM) with special effect on the leukemia and breast cancer cell lines (GI50 values <0.01 – 0.39 and 2.94 μM, respectively). Few exceptions were reported in table 1, where a small number of tumor cell lines showed a relative lower sensitivity towards the same compound (GI50 values range 10.0-59.8 μM). As concerns compound 2, it exhibited a distinctive activity against most of tested subpanel tumor cell lines with GI50 values ranging between <0.01-3.25 μM. On the other hand, the analog 3 showed an overall potential antitumor activity against all the tested cancer cell lines (GI50 values ranging between <0.01-16.1 μM). About 26 different cancer cell lines IN VITRO ANTITUMOR SCREENING OF PYRAZOLES showed obvious sensitivity pattern towards this compound, with GI50 values lying in the nanomolar concentration range (GI50 values < 0.01 μM) (Table 1). Concerning the broad spectrum of antitumor activity, the tested compounds 1-3 displayed effective growth inhibition GI 50 (MG-MID) values of 0.65, 0.19 and 0.08 μM, respectively, beside a cytostatic activity TGI (MG-MID) values of 16.9, 11.7 and 15.8 μM, respectively (Tables 2 and 3). In addition, they exhibited some cytotoxic activity with LC50 (MG-MID) values of 95.5, 87.1 and 64.6 μM, respectively (Table 4). Compound 3; 5-(1-(4-chlorophenyl)-4-hydroxy1H-pyrazol-3-yl)-2-phenyl-1,3,4-oxadiazole; having GI50, TGI, and LC50 MG-MID values of 0.08, 15.8 91 and 64.6 μM, respectively, proved to be the most active member in this study. It revealed potential activity against all the tested subpanel tumor cell lines with special high potency on the leukemia and breast cancer subpanels at both the GI50 (0.03 and 0.04 μM, respectively) and the TGI levels (27.9 and 26.2 μM, respectively) (Tables 2 and 3). Furthermore, the compound showed almost the same level of antitumor activity against the colon, CNS and prostate cancer subpanels at both the GI50 (0.2, 0.3 and 0.34 μM, respectively) and the TGI levels (11.6, 28.4 and 61.3 μM, respectively) (Tables 2 and 3). It should be pointed out that, this compound revealed a moderate cytotoxic activity against all the tested subpanel tumor cell lines with LC50 values ranging between 45.3-94.4 μM (Table 4). Table 1: Growth inhibitory concentration (GI50, μM) values of the in vitro tumor cell lines a. Cell Lines 1 2 3 CCRF-CEM HL-60 (TB) K-562 MOLT-4 PRMI-8226 SR Non-Small Cell Lung Cancer 0.25 NTb <0.01 0.39 0.23 <0.01 0.23 0.03 0.04 0.05 0.12 0.03 1.47 <0.01 <0.01 0.02 0.09 <0.01 A 549 / ATCC EKVX HOP-62 HOP-92 NCI – H226 NCI – H23 NCI – H322M NCI – H460 NCI – H522 Colon Cancer 0.93 14.2 0.47 38.7 59.8 0.32 0.33 0.24 0.37 0.05 0.03 0.09 0.15 0.07 0.07 NT 0.04 0.55 <0.01 0.13 <0.01 16.1 <0.01 0.01 0.02 <0.01 0.14 COLO 205 HCC – 2998 HCT – 116 HCT – 15 HT29 KM12 SW - 620 CNS Cancer SF-268 48.1 2.33 0.61 0.15 23.9 0.39 0.47 3.25 0.24 0.06 0.08 0.20 0.11 0.45 0.40 0.33 <0.01 <0.01 0.25 <0.01 0.51 11.8 0.08 <0.01 Leukemia Saudi Pharmaceutical Journal, Vol. 13, No. 2-3 April-July 2005 92 a b AL-SAADI ET AL SF-295 SF-539 SNB-19 SNB-75 U251 Melanoma <0.01 0.31 0.33 0.16 0.47 0.05 0.03 0.19 0.23 0.02 <0.01 <0.01 0.11 5.11 <0.01 LOX IMVI MALME-3M M14 SK-MEL-2 SK-MEL-28 SK-MEL-5 UACC-257 UACC-62 Ovarian Cancer 0.28 <0.01 0.68 27.3 0.52 0.23 0.87 <0.01 0.11 <0.01 0.13 0.54 12.0 0.11 0.43 0.10 1.63 8.34 <0.01 12.9 5.48 <0.01 0.51 0.23 IGROV1 OVCAR-3 OVCAR-4 OVCAR-5 OVCAR-8 SK-OV-3 Renal Cancer 24.2 <0.01 19.0 2.17 0.29 0.34 0.12 0.47 0.92 0.41 0.23 0.39 <0.01 0.03 3.85 0.66 <0.01 4.15 786-0 A498 ACHN CAKI-1 RXF-393 SN12C TK-10 UO-31 Prostate Cancer 0.54 0.25 NT 0.39 6.05 0.84 0.41 13.5 0.50 0.09 0.10 0.16 0.11 0.05 0.01 0.23 <0.01 4.90 <0.01 <0.01 0.28 <0.01 0.05 <0.01 PC-3 DU-145 Breast Cancer 10.0 0.33 0.35 0.06 0.36 0.32 MCF7 NCI/ADR-RES MDA-MB-231/ATCC HS 578T MDA-MB-435 BT-549 T-47D <0.01 <0.01 1.19 0.26 <0.01 2.94 0.48 0.33 0.08 3.19 0.05 0.01 0.01 0.22 0.01 0.03 1.90 0.01 0.01 2.58 0.16 The best data obtained from NCI’s in vitro disease-oriented human tumor cell screen. NT = Not Tested. Saudi Pharmaceutical Journal, Vol. 13, No. 2-3 April-July 2005 IN VITRO ANTITUMOR SCREENING OF PYRAZOLES 93 Table 2: Median growth inhibitory concentrations (GI50, μM) of in vitro subpanel tumor cell linesa. Subpanel Tumor Cell Linesb Cpd. MG- No. I II III IV V VI VII VIII IX MIDc 1 0.23 6.60 11.3 2.89 4.17 8.13 6.1 5.16 4.81 0.65 2 0.09 0.23 0.15 0.13 2.24 0.54 0.22 0.21 0.62 0.19 3 0.03 2.45 0.20 0.30 4.06 5.10 1.49 0.34 0.04 0.08 The best data obtained from NCI’s in vitro disease-oriented human tumor cell screen. I, Leukemia; II, non-small cell lung cancer; III, colon cancer; IV, CNS cancer; V, melanoma; VI, ovarian cancer; VII, renal cancer; VIII, prostate cancer; IX, breast cancer. c GI (μM) full panel mean-graph mid point (MG-MID) = the average sensitivity of all cell lines towards the test agent. 50 a b Table 3: Median total growth inhibitory concentrations (TGI, μM) of in vitro subpanel tumor cell linesa. Subpanel Tumor Cell Linesb Cpd. MGMIDc No. I II III IV V VI VII VIII IX 1 36.8 75.1 66.4 37.1 77.4 52.1 78.4 28.1 55.0 26.9 2 28.1 31.8 57.8 80.0 47.6 82.5 40.0 50.2 39.5 11.7 3 27.9 22.2 11.6 28.4 19.5 36.8 23.3 61.3 26.2 15.8 The best data obtained from NCI’s in vitro disease-oriented human tumor cell screen. For subpanel tumor cell lines, see footnote (b) of table 2. c TGI (μM) full panel mean-graph mid point (MG-MID) = the average sensitivity of all cell lines towards the test agent. a b Table 4: Median lethal concentration 50 (LC50, μM) values of in vitro subpanel tumor cell linesa. Subpanel Tumor Cell Linesb Cpd. MGMIDc No. I II III IV V VI VII VIII IX 1 ---d 94.6 96.6 37.1 85.9 --- --- --- 94.7 95.5 2 89.1 90.7 95.6 94.6 73.3 --- --- 96.6 85.5 87.1 3 94.4 69.4 45.3 81.6 57.9 80.3 72.0 82.4 68.9 64.4 The best data obtained from NCI’s in vitro disease-oriented human tumor cell screen. For subpanel tumor cell lines, see footnote (b) of table 2. c LC50 (μM) full panel mean-graph mid point (MG-MID) = the average sensitivity of all cell lines towards the test agent. d Subpanel LC50 value > 100 μM. a b Saudi Pharmaceutical Journal, Vol. 13, No. 2-3 April-July 2005 94 AL-SAADI ET AL H OH HO N HO HO N HO O S N O NH2 N N N N H PYRAZOFURIN Cl 1 H N N O N N O HO O HO N N Cl 2 On the other hand, compound 2 namely; 5-(1-(4chlorophenyl)-4-hydroxy-1H-pyrazol-3-yl)-1,3,4oxadiazol-2-one; showed nearly the same pattern of activity as 3 but to a lesser extent. It showed remarkable growth inhibitory, cytostatic and cytotoxic activities against the nine subpanel tumor cell lines as evidenced by their GI50 (MG-MID), TGI (MG-MID) and LC50 (MG-MID) values (0.19, 11.7 and 87.1 μM, respectively) (Tables 2-4). Particular activity have been shown against the leukemia subpanel at both the GI50 and TGI levels (0.09 and 28.1 μM, respectively) (Tables 2 and 3). Moreover, the compound proved to be almost equipotent Saudi Pharmaceutical Journal, Vol. 13, No. 2-3 April-July 2005 N N Cl 3 towards the non-small cell lung, colon, CNS, renal and prostate cancer subpanels at the GI50 level (0.23, 0.15, 0.13, 0.22 and 0.21 μM, respectively) (Table 2). In addition, the overall cytotoxic activity of this compound (LC50 (MG-MID) 87.1 μM) was far away from that of compound 3. Meanwhile, it is devoid of any cytotoxic potency on the ovarian and renal cancer subpanels (Table 4). Finally, compound 1; 5-(1-(4-chlorophenyl)-4hydroxy-1H- pyrazol-3-yl)-4- cyclohexyl-1,2,4-triazolin-3-thione; displayed relatively weaker growth inhibition, cytostatic and cytotoxic patterns when compared with 2 and 3 (GI50, TGI and LC50 MG- IN VITRO ANTITUMOR SCREENING OF PYRAZOLES MID values 0.65, 26.9 and 95.5 μM, respectively) (Tables2-4). Except for the pronounced activity against the leukemia subpanel (GI50 value 0.23 μM), table 2 reflected that this compound has almost the same potency against the other 8 subpanels with GI50 value ranging between 2.89 and 11.3 μM. The same range of activity was nearly maintained on the cytostatic level (TGI values 36.8-78.4 μM) (Table 3). Moreover, except for the CNS cancer subpanel, compound 1 revealed marginal cytotoxic activity and was devoid of such effect on the leukemia, ovarian, renal and prostate cancer subpanels (Table 4). The ratio obtained by dividing the full panel MG-MID (μM) of the compounds by their individual subpanel MG-MID (μM) is considered as a measure of compound selectivity. Ratios between 3 and 6 refer to moderate selectivity, ratios greater than 6 indicate high selectivity towards the corresponding cell line, while compounds not meeting either of these criteria are rated non-selective (15). All the active compounds in the present study proved to be non-selective with broad spectrum antitumor activity against the nine tumor subpanels tested with ratios ranging between 0.01-2.83 for the GI50 and 0.151.36 for the TGI. At the GI50 level, compounds 2 and 3 revealed mild selectivity towards the leukemia subpanel with selectivity ratios near 3 (2.83 and 2.67, respectively). The broad spectrum antitumor activity as well as the potential cytostatic and cytotoxic effects of such type of 4-hydroxypyrazoles will be of interest for future derivatization in the hope of finding more active anticancer lead compound(s) in the nanomolar concentration level or less. Acknowledgment The authors are very grateful to the staff members of the Department of Health and Human Services, National Cancer Institute (NCI), Bethesda, Maryland, U.S.A., for carrying out the antitumor screening. References 1. 2. 3. 4 Conclusion In conclusion, the aim of the present investigation was to present and discuss the in vitro antitumor activity of some new 4-hydroxy-1Hpryazoles carrying either the 4-substituted-1,2,4triazolin-3-thione (compound 1) or the 2-substituted1,3,4-oxadiazole (compounds 2 and 3) counterparts that are structurally related to the natural 4hydroxypyrazole; pyrazofurin. The obtained results after subjection to the consecutive three NCI screening procedures confirmed the pronounced broad spectrum in vitro antitumor activity of the 1,3,4-oxadiazole - containing compounds 2 and 3 relative to the 1,2,4-triazolin-3-thione – comprising analog 1. The proposed relationship between the chemical structure of these compounds and their biological activity has been previously discussed (13). These active three compounds at the GI 50 level exhibited TGI and LC50 levels with nearly the same order of activity; 3> 2> 1 (Tables 2 and 3). Saudi Pharmaceutical Journal, Vol. 13, No. 2-3 April-July 2005 95 5. 6. 7. 8. Sammes PG. Topics in Antibiotic Chemistry, Ellis Horwood Limited , John Wiley and Sons, New York, p. 189 (1982). Ishida J, Ohtsu H, Tachibana Y, Nakanishi Y, Bastow KF, Nagai M, Wang HK, Itokawa H, Lee KH. Antitumor Agents. Part 214: Synthesis and evaluation of curcumin analogues as cytotoxic agents. Bioorg Med Chem 2002; 10: 3481-3487. Baraldi PG, Pavani MG, Nunez M del C, Brigidi P, Vitali B, Gambari R, Romagnolia R. Antimicrobial and antitumor activity of N-heteroimmine-1,2,3-dithiazoles and their transformation in triazolo-, imidazo-, and pyrazolopirimidines. Bioorg Med Chem 2002; 10: 449456. Baraldi PG, Beria I, Cozzi P, Geroni C, Espinosa A, Gallo MA, Entrena A, Bingham JP, Hartley JA, Romagnolia R. Cinnamoyl nitrogen mustard derivatives of pyrazole analogues of tallimustine modified at the amidino moiety: design, synthesis, molecular modeling and antitumor activity studies. Bioorg Med Chem 2004; 12: 3911-3921. Storer R, Ashton CJ, Baxter AD, Hann MM, Marr CLP, Mason A.M, Mo CL, Myers P L, Noble SA, Penn HR, Wier NG, Niall G, Woods JM, Coe PL. The synthesis and antiviral activity of 4-fluoro-1-β-D-ribofuranosyl-1Hpyrazole-3-carboxamide. Nucleosides Nucleotides 1999; 18 (2): 203-216. Moukha-chafiq O, Taha ML, Lazrek HB, Vasseur J-J, Pannecouque C, Witvrouw M, De Clercq E. Synthesis and biological activity of some 4-substituted 1-[1-(2,3dihydroxy-1-propoxy)methyl-1,2,3-triazol-(4&5)ylmethyl]-1H-pyrazolo[3,4-d]pyrimidines., Il Farmaco 2002; 57: 27–32. Shen DM, Shu M, Mills SG, Chapman KT, Malkowitz L, Springer MS, Gould SL, DeMartino JA, Siciliano SJ, Kwei GY, Carella A, Carver G, Holmes K, Schleif WA, Danzeisen R, Hazuda D, Kessler J, Lineberger J, Millerd MD, Eminid EA. Antagonists of human CCR5 receptor containing 4-(pyrazolyl)piperidine side chains. Part 1: Discovery and SAR study of 4-pyrazolylpiperidine side chains Bioorg Med Chem Lett 2004; 14: 935-939. El-Gaby M SA, Atalla AA, Gaber AM, Abd Al-Wahab KA. Studies on aminopyrazoles: antibacterial activity of some novel pyrazolo[1,5-a]pyrimidines containing 96 9. 10. 11. 12. 13. AL-SAADI ET AL sulfonamido moieties. Il Farmaco 2000; 55: 596-602. Kaymakcioglu BK, Rollas S. Synthesis, characterization and evaluation of antituberculosis activity of some hydrazones. Il Farmaco 2002; 57: 595-599. Finn J, Mattia K, Morytko M, Ram S, Yang Y, Wu X, Mak E, Gallant P, Keith D. Potent and selective series of pyrazole bacterial methionyl-tRNA synthetase inhibitors. Bioorg Med Chem Lett 2003; 13: 2231-2234. Fahmy HTY, Rostom SHAF, Bekhit AA. Synthesis and antitumor evaluation of new polysubstituted thiazole and derived thiazolo[4,5-d]pyrimidine systems. Arch Pharm Pharm Med Chem 2002; 335: 213-222. Fahmy HTY, Rostom SHAF, Saudi MNS., Zjawiony JK, Robins DJ. Synthesis and in-vitro Anticancer evaluation of some new fluorinated thiazolo[4,5-d]pyrimidines. Arch Pharm Pharm Med Chem 2003; 336: 216-225. Rostom SHAF, Shalaby MA, El-Demellawy MA. Polysubstituted pyrazoles, Part 5. synthesis of new 1-(4chlorophenyl)-4-hydroxy-1H-pyrazole-3-carboxylic acid Saudi Pharmaceutical Journal, Vol. 13, No. 2-3 April-July 2005 14. 15. 16. 17. hydrazide analogs and some derived ring systems. A novel class of potential antitumor and anti-HCV agents. Eur J Med Chem 2003; 38: 959-974. Grever MR, Schepartz SA, Chabner BA. The national cancer institute cancer drug discovery and development program. Seminars Oncol 1992; 19: 622-638. Boyd MR, Paull KD. Practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Rev Res 1995; 34: 91-109. Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Jangley J, Cronisie P, Viagro-Wolff A, Gray-Goodrich M, Campell H, Boyd M. Feasibility of a high flux anticancer drug screen utilizing a derive panel of human tumor cell lines in culture. J Natl Cancer Inst 1991; 83: 757-766. DeVita VT Jr, Hellman S, Rosenberg SA. Cancer Principles and Practice of Oncology, 6th Ed., Lippincott Williams and Wilkins, Philadelphia, USA, p. 349 (2001).