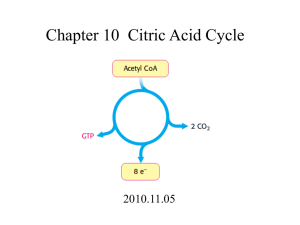
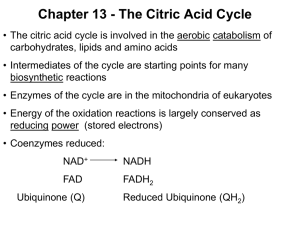
The TCA Cycle
advertisement

FUNDAMENTALS 1 10:00-11:00 THURSDAY, AUGUST 26TH, 2010 POPOV I. TCA CYCLE Scribe: TERRI CALL Proof: BRANDON TERRY Page 1 of 6 Slide with Formulas (not in handout) [S--]: a. b. He doesn’t want us to memorize the formulas as far as he is concerned, but you may have noticed that every time he has shown us a reaction, he has also shown us the ΔG value (or free-energy change) for the reaction. It’s not for us to memorize, but for us to get a better grip of what is going on: for example, where the equilibrium is. c. So if the reaction has a very negative ΔG, it means that the equilibrium is towards the product; if there is a very positive ΔG, it means that the equilibrium is toward the initial reactants. This allows you to predict how this reaction goes in real life. For example, if someone says that a reaction goes from A to B but equilibrium indicates that everything is A, you would know that you would need to get a further explanation of the reaction because that would be unusual. d. The second benefit of ΔG is that you can use it to predict which steps in a pathway are regulated. Regulation usually targets steps that have a very highly negative ΔG, because those reactions are usually far from equilibrium. If you’ll recall from the description of glycolysis, three of the reactions given are regulated reactions; this is important in order to understand how phosphate directs the reactions. e. There is a slight issue with ΔG, though, because it’s standard free-energy change. How chemists define standard condition is: you take all the reactants with a concentration of 1M, throw them in a bucket, adjust the pH to 1.0, fix the temperature at 25°, let it sit until it reaches equilibrium, measure the concentration of the reactants, define and determine the equilibrium constant, and then you get your standard free energy change. This is far away from what happens in real life, since there is no concentration of reactants in the cells that are either 1M or pH 1.0. f. So in reality, we use a value called standard free-energy change adjusted. This uses a pH of 7.0, since it’s more realistic. However, we still use 1M concentration. g. There is a simple equation that relates standard free energy change to the standard free-energy change that occurs at the current concentration (the second equation given above). In general, if you do the math, you’ll see that the values usually turn out to be even greater; for example, the standard free energy of ATP hydrolysis is about -30 kJ, but in the cell it will be -70 kJ. It doesn’t compromise the worth of the standard value found since they are still pretty good and predictable, but we should keep in mind that in real life the values will be disparate. II. Title Slide [S1] a. Today we are discussing the TCA Cycle (aka “tricarboxylic acid cycle” or “citric acid cycle”). III. Overview of Lecture [S2] a. We will discuss the source of acetyl-CoA (because this is a pathway that is an endpoint for all nutrients that we oxidize for energy), the enzymes and reactions of the citric acid cycle, how the pathway is regulated, and a little bit of the other aspects of the reaction since this cycle is often perceived as simply a pathway to burn carbon in mitochondria, but in real life it has other functions that are important to keep in mind. IV. Figure, Stages of cellular respiration [S4] a. This slide summarizes the three stages of catabolic reactions that oxidize the available sources of carbon. b. There are three major metabolic fuels: carbohydrates, which are primarily glucose; fat, which is primarily fatty acids; and amino acids, which are derived from the digestion of proteins. Their values are somewhat different as we use them in different ways, but we should realize that all of them can be burned to eventually get CO 2, and the energy that is released from that burning can be used to drive biochemical reactions, muscle and neural activity, and so forth. c. Yesterday, we were focusing primarily on the preparatory stage where the skeletons of carbs were broken down to pyruvate in a sequence of reactions called glycolysis. Later in the course, we will look at a sequence of reactions that lead to the breaking down of fatty acids, or beta-oxidation reactions. The outcome of those reactions will be the conversion of fatty acids to acetic acid; acetic acid doesn’t exist free but in the cell in its activated form, connected to acetyl CoA. This is the universal carrier of acyl groups in cells. As a general rule, most of them will be broken down to pyruvate, acetyl CoA, or sometimes to certain intermediates of the oxidative cycle. d. In this cycle, complex molecules are broken down into simpler molecules: carbs to pyruvate, fatty acids to pyruvate and acetyl CoA. Eventually, all the carbon then ends up in the form of mitochondrial acetyl CoA, and it’s oxidized in one common pathway called the citric acid cycle. e. The cyclic pathway’s outcome is that acetyl CoA is oxidized, carbon is released in the form of CO 2, and electrons that have been obstructed during the oxidation reactions are released in the form of electron FUNDAMENTALS 1 10:00-11:00 Scribe: TERRI CALL THURSDAY, AUGUST 26TH, 2010 Proof: BRANDON TERRY POPOV TCA CYCLE Page 2 of 6 carriers which are primarily NADH and FADH2. In the last stage of oxidation, all the electrons are fed to the electron transport chain and eventually donated to oxygen. The energy of the electron current is then used to make ATP, which is the topic of the next hour’s lecture. V. Figure, Compartmentalization of Glycolysis, the Citric Acid Cycle, and Oxidative Phosphorylation [S4] a. As far as oxidation of carbs goes, you’ll recall that most of the preparatory stage of changing glucose to pyruvate takes place in the cytosol. This is a unique feature of carbohydrates, as fatty acids and amino acids will be oxidized directly in the mitochondria. b. The ability of glucose to be oxidized in the cytosol is unique. It allows some energy to be produced from the oxidation of glucose even when there is no mitochondria present in the cell, which is very useful in some areas of the body like the cornea. c. Before carbs can be completely oxidized in the citric acid cycle, pyruvate must be taken inside the mitochondria and converted into acetyl CoA (before it enters the citric acid cycle). VI. Figure, Glycolysis is a Preparatory Pathway for Aerobic Metabolism of Glucose [S5] a. Everything that takes place in mitochondria is dependent on oxygen. Some reactions of the citric acid cycle don’t require oxygen, like the pyruvate dehydrogenase reaction, but the mitochondria require it in order to function and get rid of NADH accumulations that occur during these reactions. b. Because of this, when there is no oxygen, all processes in mitochondria will stop. c. This is another reason carbs are unique, since they can be used under anaerobic conditions. This is particularly important for muscle contractions since that obstructs blood flow and reduces oxygen in the area. During this time, it cannot oxidize fatty acids or amino acids, so most of the energy used to support muscle activity during these times comes from glycolytic phosphate. d. In the first step on this slide, pyruvate is converted to acetyl-CoA. This is a reaction catalyzed by pyruvate dehydrogenase (aka pyruvate dehydrogenase complex). This is not part of the citric acid cycle, but we can view it as a preparatory step for the carbohydrates to be able to enter the citric acid cycle. It operates in close conjunction with the citric acid cycle itself, though, since it is a way to feed the carbon from glucose into the cycle. VII. Overall reaction catalyzed by pyruvate dehydrogenase complex [S6] a. The pyruvate dehydrogenase reaction takes pyruvate in and produces acetyl CoA and CO 2. So, this is technically an oxidative decarboxylation reaction since CO 2 is released. In the course of the reaction, the keto group (the double-bonded O on the middle carbon in pyruvate) is oxidized into acetic acid. b. The standard ΔG here is very highly negative, which tells us that the reaction is nearly irreversible. This is for good reason, since this drives products in a particular direction in a variety of systems. (Nearly all carboxylation reactions are irreversible.) In this instance, the reason is simple; the CO2 released is a gas and when it leaves the reaction, it almost immediately reacts with water, becoming bicarbonate. This continuously removes products of the reaction from the media and moves the process in one direction. c. However, even though ΔG is high, we still don’t waste the energy of the carboxylation; it is stored in reduced NADH and acetyl CoA, which are high-energy compounds. This is important because it will be driving the actions of the citric acid cycle, which also proceeds in one direction. d. Lastly, although this reaction appears simple, it requires coenzyme A, NAD, TPP, lipoate, and FAD, which tells you in reality that it is a multi-enzyme complex, or an assembly of several enzymes working closely with each other. VIII. Coenzyme A (CoA) [S7] a. To remind you: CoA is a carrier of acyl or acetyl groups. In this case, it reacts with acetic acid to form an acetyl coenzyme intermediate. Like NADH is a carrier of electrons, this is a carrier of acetyl or acyl groups if we’re talking about fatty acids. b. On the molecule shown, the pantothenic acid (an actual vitamin) is needed primarily for recognition. IX. Lipoic Acid (Lipoate) in Amide Linkage with Lys Residue [S8] a. Lipoic acid is a prosthetic group that is covalently bonded to one of the enzymes of the multi-enzyme complex. It is a very long arm that actually moves intermediates from one active site to another in the complex. b. The ring at the top of the molecule (with the two Ss) can be oxidized to be closed or reduced to be open. It is similar to CoA in that it is a carrier of acetyl groups in this particular enzyme. The whole thing grabs acetic acid and moves it from one active site to another. c. Pyrophosphate in this set of reactions is a coenzyme that assists in the catalysis of the first decarboxylation reaction, which is what makes the whole process irreversible. X. Structure of the Pyruvate Dehydrogenase Complex [S9] a. The pyruvate dehydrogenase complex in reality is pretty large. In size, it’s similar to ribosomes. It consists of multiple copies of three enzymes, E1, E2, and E3, and the whole thing is arranged in such a way that FUNDAMENTALS 1 10:00-11:00 Scribe: TERRI CALL THURSDAY, AUGUST 26TH, 2010 Proof: BRANDON TERRY POPOV TCA CYCLE Page 3 of 6 intermediates are moving from one active site to another until the final product is made and released into the media. b. The intermediates do not leave the enzyme at all, just continuously attach to different parts of the enzyme complex. XI. Figure, Oxidative Decarboxylation of Pyruve to Acetyl-CoA by the PDH Complex [S10] a. In reality, it consists of five enzyme-catalyzed reactions. b. The first is catalyzed by E1, thiamine pyrophosphate (TPP). TPP takes pyruvate in and reacts it with thiamine. Thiamine attacks the alpha carbon of pyruvate, causing the release of CO 2, and causing the formation of an intermediate that is covalently attached to TPP. All this happens in the active site of E1. c. In the next stage, lipoic acid attached to E2 comes into the active site of E1, and covalently attaches the intermediate to itself, forming an aldehyde. This is a high-energy intermediate similar to acetyl CoA. This oxidation reaction during transfer happens because lipoic acid enters the reaction in its oxidized form and leaves it in its reduced form. It happens because the aldehyde becomes oxidized in the acid; the lipoic acid is reduced by electrons from the aldehyde. When this happens, the whole thing leaves the active site of E1 and enters the active site of E2, which catalyzes the transacetylation reaction, moving the acetyl group from lipoic acid to the incoming CoA. d. Remember, the molecules in the pink boxes at steps 2 and 3 are high-energy intermediates, so energy is not wasted during this transfer (an equilibrium reaction). Lipoic acid leaves the reaction in the reduced state, which means that the enzyme cannot enter the next round of catalysis, since it leaves the stage in the wrong oxidation state. The next several reactions, then, are meant to restore the catalyst itself. e. The fourth reaction actually takes place in the active site of E3, where lipoic acid is oxidized back and the electrons are moved to flavin nucleotide, which is tightly bound to E3. f. The enzyme is now ready for catalysis, but FAD is still in reduced form. Therefore, in the last stage of catalysis, this prosthetic group is oxidized back to FADH2 with the assistance of NAD (reduced in the process). g. The overall outcome of the cycle will be the carboxylation of pyruvate, producing acetyl CoA and one molecule of reduced electron carrier NADH. h. **NOTE: “important for later consideration.” This reaction is irreversible, which creates many problems studying metabolism and its regulation in humans and animals. Plants, though, can actually synthesize pyruvate out of acetyl CoA with specialized enzymes. So if the plant runs out of glucose for some reason, it can mobilize fatty acids to make carbs and doesn’t have any problems. Unfortunately for us, we can’t convert our stored triglycerides to glucose since we don’t have an apparatus to convert them. And since some of our tissues rely solely on glucose for energy, this is why we need to go into the conservation of glucose when we go into starvation; this is why we break down proteins when starving. This is all primarily due to this reaction being irreversible, since if it was reversible we could use it to make sugars in starvation conditions. XII. Figure, Reactions of the Citric Acid Cycle [S11] a. We produce acetyl CoA in this particular case out of glucose. But it can also be produced from fatty acids and amino acids with different enzymes. b. Now it can enter the citric acid cycle. The first thing to notice is that it is a cycle that begins with the condensation of oxaloacetate and CoA, although the CoA is recovered during the turn of the cycle. It also tells you that oxaloacetate works as a catalyst of the process, entering the sequence and leaving it unchanged. Theoretically, one molecule of oxaloacetate is sufficient to oxidize an enormous amount of acetyl CoA, since it operates as a catalyst. This is also important because mitochondrial concentrations of oxaloacetate are very low, and the cell goes to a lot of trouble to maintain mitochondrial oxaloacetate. c. Equilibrium of this reaction is towards malate (end product, #8) though there is not that much of it made. d. There is very little oxaloacetate in mitochondria, so if it were to escape the mitochondria to the cytosol directly, the whole cycle would stop. Consequently, the mitochondrial membrane is sealed against oxaloacetate so it can’t escape. (However, oxaloacetate can be used in synthetic pathways or as a shuttle; since it can’t be transported directly, it has to be converted into some other molecule that can be taken out of the mitochondria and then converted back. However, if you take it out of the mitochondria, it must be replenished, and there are several enzymes that can do this.) e. The cycle takes two carbons in, and then, after a few steps, puts two carbons out. This tells you that the two carbons are oxidized in the course of this reaction; acetate, therefore, is completely oxidized. (The carbons that enter the cycle are not the same as the carbons that are released on the first turn—they’re in different positions, and this has to do with the iconitase reaction. So it takes several turns of the cycle to get rid of the carbons that were incorporated into the oxaloacetate.) f. So the output of one turn is: two carbons out, several different reduced electron carriers, primarily NADH and one FADH, as well as one molecule of ATP made directly in the course of substrate-level phosphorylation. So most of the output of the cycle is not energy itself, but reduced electron carriers. FUNDAMENTALS 1 10:00-11:00 Scribe: TERRI CALL THURSDAY, AUGUST 26TH, 2010 Proof: BRANDON TERRY POPOV TCA CYCLE Page 4 of 6 XIII. Formation of Citrate [S12] a. Now let’s take a look at the reactions associated with this cycle. Acetyl CoA enters the cycle through the reaction catalyzed by citrate synthase. It has a very large negative ΔG change, which makes sense since acetyl CoA is a high-energy compound broken down over the course of the reaction. Consequently, the reaction is putting phosphate in a particular direction—towards citrate. b. The product of this reaction is a citrate, so carbon will be oxidized in the following reactions. It’s a tertiary alcohol, which is very difficult to oxidize because they’re not very reactive. This is why there are several reactions that precede oxidation, converting citrate to the secondary alcohol isocitrate, to make a better substrate for oxidation. XIV. Formation of isocitrate via cis-aconitate [S13] a. This is catalyzed by iconitase. This is actually an energetically unfavorable reaction, so for the process to continue in that direction, there must be steps further down the line that pull the product in that direction. b. So iconitase forms aconitate as an intermediate, flipping the marked H and OH groups in position in the process. It’s much easier to oxidize isocitrate rather than citrate. XV. Oxidation of isocitrate to α-ketoglutarate and CO2 [S14] a. Oxidation of isocitrate takes place in the isocitrate dehydrogenase reaction. It’s virtually irreversible. b. It’s not only the oxidation of isocitrate, it also is a carboxylation reaction, which is irreversible. This is why the equilibrium is still towards substrates with this reaction; by pulling the isocitrate towards α-ketoglutarate, it pulls over all products towards oxidative degradation of isocitrate. XVI. Oxidation of α-ketoglutarate to succinyl-CoA and CO2 [S15] a. α-ketoglutarate is subjected to oxidative decarboxylation again. The α-ketoglutarate dehydrogenase makes succinyl CoA here, an enzyme very similar to pyruvate dehydrogenase here. It’s a very similar multi-enzyme complex that uses enzymes 1, 2, and 3, and uses the same pyrophosphate and FAD. It is also virtually physiologically irreversible. The output is succinyl-CoA, which is a high-energy compound that can be used to make ATP. XVII. Conversion of succinyl-CoA to succinate [S16] a. ATP is made in this reaction. b. The reaction is called succinyl-CoA synthase; under most physiological conditions it creates GTP and converts it to succinate. c. By this time, we have already oxidized all carbons and all reactions downstream from here are just meant to replenish acetyl CoA. d. This is one of those substrate-level phosphorylations that are used to make high-energy compounds without participation in the electron transport chain. XVIII. Succiny-CoA synthase reaction [S17] a. The succinyl-CoA is a high-energy compound that can react with phosphate and the active site of an enzyme to form a phosphoryl intermediate. b. Next step is a phosphoryl group transferred to a histidine residue on the enzyme, releasing succinate. The high-energy intermediate immediately donates its phosphate to GDP, producing GTP. All nucleotides are related metabolically, so it can be readily converted to ATP. XIX. Oxidation of Succinate to Fumarate [S18] a. The next step is catalyzed by the oxidation of succinate to fumarate (succinate hydrogenase reaction). b. Note that the electron carrier here is FAD because this compound does not have enough energy to reduce NAD. This is the only enzyme of the citric acid cycle that is attached to that membrane; a lot of the enzymes are soluble in the mitochondrial matrix, but this one is a part of the membrane itself, and as we will see in the next lecture, it is not only part of the citric acid cycle, but also the electron transport chain. This enzyme will be called Complex II there. XX. Hydration of fumarate to malate [S19] a. Fumarate is hydrated and converted to malate. b. The reaction is catalyzed by fumarase. XXI. Oxidation of malate to oxaloacetate [S20] a. In the last stage, malate dehydrogenase oxidizes malate into oxaloacetate. b. Several reactions we have described will have very similar chemistries in metabolic pathways as reactions we will study later in the course, such as beta oxidation. XXII. Products of one turn of the citric acid cycle [S21] a. This finalizes one turn of the cycle. To sum it up, 2 carbons go in, 2 carbons go out, electron carriers are produced, and one high-energy phosphate is produced. XXIII. Table, Stoichiometry of Coenzyme Reduction and ATP Formation in the Aerobic Oxidation of Glucose via Glycolysis, Pyruvate Dehydrogenase Reaction, the Citric Acid Cycle, and Oxidative Phosphorylation [S22] FUNDAMENTALS 1 10:00-11:00 Scribe: TERRI CALL THURSDAY, AUGUST 26TH, 2010 Proof: BRANDON TERRY POPOV TCA CYCLE Page 5 of 6 a. This is a summary of the things we have discussed so far. These are high-energy compounds invested in the early stages of glycolysis up to this point, electron carriers, and in the last column is the number of molecules of ATP that can be produced out of each compound. b. We don’t have to memorize these numbers, but one useful number to know is that from the oxidation of ONE molecule of glucose, the cell can make up to THIRTY-TWO molecules of ATP. (This is not as good as oxidizing fatty acids, which release much more energy during oxidation because there, the glucose is already partially oxidized.) XXIV. Role of the citric acid cycle in anabolism [S23] a. An important point about the citric acid cycle is that historically, it is discussed as a major degradative pathway, but in reality it’s also used in a variety of biosynthetic processes. b. This slide discusses where various intermediates of the cycle are used; for example, citrate can be used as a precursor for biosynthesis of fatty acids or cholesterol in cytosol, so citrate produced in the first citrate synthase can be taken out of the mitochondria into the cytosol and broken down by acetyl CoA, where it can be used in biosynthetic reactions. c. α-ketoglutarate is precursor of glutamate which can be used to synthesize purines and other amino acids. d. Succinyl-CoA is a major precursor of porphyrins like heme and cytochromes. e. Oxaloacetate is a precursor of amino acids and pyramidines and can be used in biosynthesis of glucose and gluconeogenesis. f. It’s not just degradative phosphate but also biosynthetic phosphate, so it’s sometimes referred to as amphibolic phosphate (used in both catabolic and anabolic processes). g. NOTE: If you throw the citric acid out of cycle, it’s not just removing citric acid out of the cycle, it’s also removing oxaloacetate. Consequently, the entire citric acid cycle will stop because there is no oxaloacetate anymore. If this happens, biosynthetic reactions will stop, since they will have no energy. So you must replenish mitochondrial oxaloacetate, and there are several reactions which serve this purpose. XXV. Table, Anaplerotic Reactions [S24] a. The most important one is catalyzed by pyruvate carboxylase, which carboxylates pyruvate to produce oxaloacetate. XXVI. The role of biotin in pyruvate carboxylase reaction [S25] a. Pyruvate carboxylase is a classic example of a biotin-dependent enzyme. It’s actually used as a prosthetic group of pyruvate carboxylase. b. It’s attached to the amino group of lysine, so it forms a very long, swinging arm that can move a carboxyl group from one active site to another. c. The carboxylation reactions involve CO2, and under physiological conditions there is almost no free CO2 in our system as it’s all present in the form of bicarbonate (a result of carbonic acid dissociating to form bicarbonate). So before the enzyme can carboxylate anything, it must convert bicarbonate into CO 2, an unfavorable reaction that requires ATP. This happens in one active site of the enzyme. d. After the CO2 has been made, we must be able to keep hold of the gas, or it will react with water and turn back into bicarbonate. This is where biotin comes into play; it directly reacts with CO 2 to form a covalent intermediate that prevents it from escaping. This intermediate moves from one active site to another where the CO2 is released and directly interacts with pyruvate from acetyl-CoA. e. So biotin is a coenzyme of carboxylation reactions because it can hold CO 2 and prevent it from reacting with other things. XXVII. Regulation of PDH Complex [S26] a. As we’ve seen, there are many ways to regulate the reactions of the citric acid cycle, but it’s important to realize that the primary step to control the cycle is by controlling the rate at which carbon is fed into the citric acid cycle. b. So the primary control of the metabolism of carbs is not the citric acid cycle itself, but by regulating the reactions that feed carbon into the cycle, the reaction catalyzed by pyruvate dehydrogenase complex. This is the major site of regulation of activity of citrate. c. So you should regulate the supply of carbon into the cycle. d. Consequently, the regulation of pyruvate dehydrogenase complex is really complicated. e. There are allosteric and phosphorylation mechanisms that should be considered. A rule of thumb is that the allosteric processes are more important in bacteria; in mammals, it’s the phosphorylation processes. f. Besides all of those enzymes, each complex has dedicated protein kinase and protein phosphatase. The kinase catalyze the phosphorylation of the E1 enzyme and makes it completely inactive; the dedicated phosphatase removes the phosphate and makes it active again. Most kinases and phosphatases are regulatory enzymes. g. The rule of thumb is that the products of the pyruvate dehydrogenase reaction activates the kinase; you build up product, and it is not consumed in the citric acid cycle, so it tells the enzyme that it’s time to slow down the FUNDAMENTALS 1 10:00-11:00 Scribe: TERRI CALL THURSDAY, AUGUST 26TH, 2010 Proof: BRANDON TERRY POPOV TCA CYCLE Page 6 of 6 feeding of carbon. Kinase becomes active, kills the enzyme; products are consumed, kinase activity goes down, phosphates dephosphorylates the enzyme, and feeding is resumed. This is the main way to control the activity of the citric acid cycle as far as the metabolism of carbs is concerned. XXVIII. Regulation of metabolite flow through the citric acid cycle [S27] a. There are other methods of regulation, but this is a more minor regulation that does not have much bearing on most of the physiological conditions of the body. XXIX. Conclusions [S28] a. No lecture given for this slide.