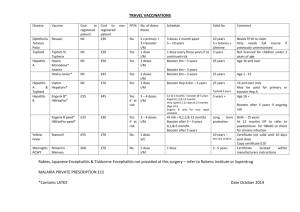
הודעה על החמרה ( מידע בטיחות) בעלון לצרכן

תוחיטב עדימ ( הרמחה לע העדוה
27.4.2009
_______ ךיראת
Infanrix
®
______תילגנאב רישכת ם ש
104-10-28630
__________ םושיר רפסמ
GlaxoSmithKline (Israel) Ltd., 25 Basel St., Petach-Tikva
םושירה לעב
בוהצ עקר לע םינמוסמ ןולעב םייונישה
49002
אפור ל ל ןולעב )
שדח טסקט
םי/שקובמה םי/יונישה לע םיטרפ
יחכונ טסקט ןולעב קרפ
Clinical trials:
The safety profile presented below is based on data from more than 11400 subjects.
As has been observed for DTPa and
DTPa containing combinations, an increase in local reactogenicity and fever was reported after booster vaccination with Infanrix with respect to the primary course.
Frequencies per dose are defined as follows
Very common:
10%
Common:
1% and < 10%
Uncommon:
0.1% and < 1%
Rare:
0.01% and < 0.1%
Very rare: < 0.01%
Blood and lymphatic system disorder s
Very rare: Lymphadenopathy 1
Metabolism and nutrition disorder
Common: appetite lost 2
Psychiatric disorders:
Very common: irr itability
Common: restlessness 2 , crying abnormal
Nervous system disorders:
Very common: somnolence
Uncommon: headache 1
Respiratory, thoracic and mediastinal disorders:
Uncommon: cough 1 , bronchitis 1
Gastrointestinal disorders:
Common: gastrointestinal disor ders such as diarrhoea and vomiting
Skin and subcutaneous tissue disorders
Common: pruritu s
Uncommon: rash
Rare: urticaria
General disorders and administration site conditions:
Very common: redness, local swelling at the injection site (≤50 mm),
38.0°C
, fever
Common: pain 2 , local swelling at the
In controlled clinical studies, signs and symptoms were actively monitored and recorded on diary cards in all vaccinees following the administration of each dose of the vaccine.
The following table, based on the results of comparative studies summarises the local solicited symptoms reported within 48 hours of vaccination as a percentage of doses administered.
Local Solicited Primary
Symptoms immunization
INFANRIX TM DTPW
(1275 doses) (455 doses)
Pain 2.5 19.1
redness (>2cm) 0.1 1.1
swelling (>2cm) 0 1.3
BOOSTER
INFANRIX
TM
DTPW INFANRIX
TM
DTPW after after after after
INFANRIX
TM
DTPW DTPW DTPW
Primary primary Primary primary
(269 doses) (92 doses) (273 doses) (91 doses)
15.6 55.4 15.8 59.3
4.5 3.3 2.2 5.5
3.9 7.6 1.5 5.5
General solicited symptoms which were reported in the same comparative studies and within the same timeframe are summarised in the following table.
General solicited Primary
Symptoms (%) immunization
INFANRIX TM DTP fever
38
C (rectal) 9.9 42.2
fever
39.5
C 0.2 1.3
W
(rectal)
unusual crying 5.2 11.9 vomiting 3.0 4.4 diarrhea 5.9 6.8 eating and drinking l4.2 20.7 less than usual sleeping more than 9.3 13.6
usual / drowsiness sleeping less than 9.31 16.7 usual / restlessness
Additional safety data are available from other studies, which evaluate the primary immunisation course and the booster dose administration.
Adverse
Reactions
(events)
injection site (>50 mm) 3
Uncommon: injection site reactions including indurations, fatigue 1 , fever
39.1°C
, diffuse swelling of the injected limb, sometimes involving the adjacent joint.
3
Post marketing sur veillance:
Blood and lymphatic system disorders:
Thrombocytopenia 4
Immune system disorders :
Allergic reactions, including anaphylactic and anaphylactoid reactions
Nervous system disorders :
Collapse or shock-like state
(hypotonic-hyporesponsiveness episode ), convulsions (with or witho t fever) within 2 to 3 days of vaccination
Respiratory, thoracic and mediastinal disorders:
Apnoea [see section “Warnings and
Precautions” for apnoea in very premature infants (≤ 28 weeks of gestation)]
Skin and subcutaneous tissue disorders:
Angioneurotic oedema,
General disorders and administration site conditions :
Swelling of the entire injected limb 3
1 reported only with booster vaccination
2 very common for booster vaccination
Wording for those that do not have a booster indication in children ≥ 4 years of age:
3 Children primed with acellular pertussis vaccines are more likely to experience swelling reactions after booster administration in comparison with children primed with whole cell vaccines. These reactions resolve over an average of 4 days.
Wording for those having a booster
These studies, which include noncomparative studies, confirmed the safety profile of DTPa which is summarized above.
Studies have been conducted to evaluate the incidence of local swelling reactions after booster administration. The frequency of these reactions was as follows:
Very common (= 10%): local swelling at the injection site (≤ 50 mm)
Common (=1/100, <1/10): local swelling at the injection site (> 50 mm)*
Uncommon (=1/1,000, <1/100): diffuse swelling of the injected limb, sometimes involving the adjacent joint.*
*Children primed with acellular pertussis vaccines are more likely to experience swelling reactions after booster administration in comparison with children primed with whole cell vaccines. Local swelling at the injection site (>50 mm) and diffuse swelling may be more frequent (very common and common, respectively) when the booster dose is administered between 4 and 6 years.
These reactions resolve over an average of 4 days.
The following unsolicited symptoms have been reported for:
INFANRIX TM primary immunisation
(total of 11406 documented doses):
Skin and appendages (1% or less): dermatitis
Respiratory system (3% or less): coughing, rhinitis, bronchitis, other upper respiratory tract infection.
indication in children ≥ 4 years of age:
3 Children primed with acellular pertussis vaccines are more likely to experience swelling reactions after b ooster administration in comparison with children primed with whole cell vaccines. Local swelling at the injection site (>50 mm) and diffuse swelling may be more frequent (very common and common, respectively) when the booster dose is administered between 4 and 6 years. These reactions resolve over an average of 4 days.
4 reported with D and T vaccines
Resistance mechanism (1% or less): otitis media
INFANRIX
TM
booster following
INFANRIX TM primary immunisation
(total of 2363 documented doses):
Respiratory system (4% or less): coughing, pharingitis, bronchitis, other upper respiratory tract infection, rhinitis, respiratory disorder
Resistance mechanism (3% or less): viral infection, otitis media
INFANRIX
TM
booster following
DTPw primary immunisation (total of 606 documented doses):
Respiratory system (3% or less): coughing, pharingitis, upper respiratory tract infection, bronchitis.
Resistance mechanism (2% or less): otitis media.
Post-marketing surveillance:
Very rare allergic reactions, including anaphylactoid reactions, have been reported.
Extremely rare cases of collapse or shock-like state (hypotonichyporesponsiveness episode) and convulsions within 2 to 3 days of vaccination have been reported. All the subjects recovered totally and spontaneously without sequelae.
Swelling of the entire injected limb.
Drug
Interactions
Excipients: Removal
2 P henoxyethanol
Overdosage
Not applicable.
Cases of overdose have been reported during post marketing surveillance.
Adverse events, when reported, are not specific but similar to adverse events reported with normal vaccine administration .
Storage
Addition:
The vaccine should be administered immediately after opening of the vial (not later than 8 hours after opening).
Overdosage
Not applicable.
Storage
2-Phenoxyethanol