Safety Services
advertisement

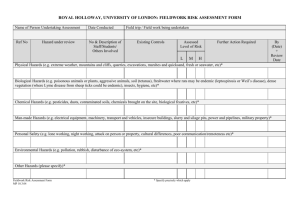
SCBS risk assessment form for project reference no: ................................ (Insert ref. number as allocated by SCBS) Sub Committee for Biological Safety Human and Environmental Risk Assessment for activities involving the Contained Use of genetically modified organisms Before completing this form please read the notes for guidance below. The Biological Safety Officer [ext. 8887, or m.iosson@rdg.ac.uk] may be consulted for advice and further information. Notes for Guidance, and where to obtain further help. A copy of this form must accompany each new GM project proposal (form GM1): the aim is to assist you when writing the Lay Summary for assessment by SCBS. It should also be used in the annual review of each GM project. See Safety Guide 15 (3rd edition) for general advice of GM procedures at The University, including activity classification. You should also consult the ACGM Compendium of Guidance, available from the HSE website, http://www.hse.gov.uk/biosafety/gmo/acgm/acgmcomp/ Possible hazards For Part A, question 1: include any toxic, allergenic or other pathogenic effects that could be caused by the GMM on humans and/ or other organisms. Take into account the levels of expression expected in the GMM, and the source of the gene/ sequence being expressed. For “antisense” RNA transcripts, identify function of gene(s) interfered with and indicate possible effects on the resultant GMO. Take into account any possible need for an inducer to initiate protein production. Do not forget factors such as antibiotic resistance markers in plasmid vectors! For genetically modified viruses or viral vectors, include possibility of recombination with unmodified or related viruses infecting the same target cell. Other hazards include colonisation of the human or other organisms by the GMM; ability to survive in the environment; competition with organisms in established ecosystems; ability of the GMM to transfer genes to other organisms, etc. For Part B, question 8: Possible hazards are similar to those of GMMs, with the exception of infection. The assessment must include possible hazards to humans (e.g., toxic or allergenic effects) as well as hazards to the environment. For GM plants, particular attention should be paid to dissemination of seeds, pollen, etc., and to the products of gene expression, especially if they are toxic or potentially allergenic. Phenotypic and/ or genotypic stability may also be important, especially if loss or movement of the inserted genetic material could have other consequences, such as altering the expression of other genes within the plant. If the inserted genetic material includes antibiotic resistance or herbicide tolerance genes as markers, any environmental effects of marker transfer to other organisms must be included. When assessing likelihood (Part A, Q2;) and Part B, Q9 take into account the properties of the GMO, the scale of operations, and pay particular attention to any techniques that are likely to increase exposure of individuals to GMMs (e.g., operations creating aerosols.) The likelihood of these hazards being realised will depend on the nature of the receiving environment. In order to interpret the term “posing no greater risks to humans than the non-modified parent" (formerly “as safe in containment as the non-modified parent, “ASIC”), applicants need only consider the effects of the modifications on the properties [including “behaviour”, if appropriate] of the organism that has been modified. Whether the recipient or parental organism itself is or is not “safe” in containment is irrelevant to the classification, but it must not be forgotten when making any arrangements for supervision of the work. Risk Assessment matrix: The following table should be used when assessing the magnitude of the risk from the likelihood and the consequences of a hazard being realised. (Risk = likelihood x consequence). The term "nonzero risk" is used for those risks over and above the magnitude of "effectively zero". GM03-11-2007 Form design M.Iosson 2007 Page 1 SCBS risk assessment form for project reference no: ................................ (Insert ref. number as allocated by SCBS) Likelihood of hazard being realised Medium Low Negligible Consequence of hazard being realised High Severe (e.g., danger of death or major effects on human health or ecosystems) Medium (e.g., major effects on a few individuals) Low (Only minor effects) High High Medium Effectively zero High Medium Medium/ Low Effectively zero Medium/ Low Low Low Effectively zero Negligible (Possible effects hard to detect) Effectively zero Effectively zero Effectively zero Effectively zero Unless the risk is "Effectively zero" under conditions equating to Containment level 1, additional containment measures will be required to reduce the risk to "Effectively zero". The nature of the additional containment measures determines the Risk Class for the GMM (see below.) Thus, for example, a GMM which is derived from an animal pathogen normally requiring Containment level 2 should be assessed as having a low to medium consequence if it were to escape from containment and infect its normal host species. This would apply, unless there is definitive evidence that the GMM is non-pathogenic, and unlikely to cause any other effects in the target species (i.e., consequence is "negligible"). In the absence of such evidence, it must be assumed that the GMM may still be pathogenic, and have low - medium consequence if it were to escape. The likelihood of escape would depend on the containment level applied. If the consequence is "negligible", containment level 1 would suffice, but otherwise, the risk can only be reduced to "effectively zero" by applying additional containment measures. The nature of the additional measures helps to determine the risk class (see below.) Assignment of Risk Class (for GMMs). The risk class for GMMs is equal to the level of the containment required to control the risks of the activity. For example, consider the situation when a microbiological safety cabinet (or other equipment) is required to control infectious aerosols, but it is not necessary to have the laboratory sealable for fumigation. This is a characteristic of Containment level 2, hence the activity would be classified as Class 2. This is irrespective of whether the GMM is infectious for humans, or it could have a moderate environmental impact if it were to escape from containment. GM03-11-2007 Form design M.Iosson 2007 Page 2 SCBS risk assessment form for project reference no: ................................ (Insert ref. number as allocated by SCBS) Risk assessment and classification Where this form is used for the Annual Review of the risk assessment, highlight any changes made since the last risk assessment form for this project was submitted. Please complete Part A for GMMs, and / or Part B for GMOs that are not microorganisms. You will need to complete both parts of this section if your project involves both GMMs and higher organisms. Please delete part A if not applicable Part A: Activities with genetically modified microorganisms Use the information in the project proposal form GM1 to answer the questions posed below. You must justify any statements made, e.g., by references to the scientific literature where necessary. Please ensure that you include consideration of those aspects of the proposed work that will carry the greatest risk. 1. Identify all potentially hazardous properties of each GMM. Do not forget hazardous properties of the parental organism. Consider ALL properties of the host, vector, insert, and of the final GMM. Use one entry for each type of GMM. Separately list hazards to human health and safety, and hazards to the environment. A: Hazards to Human health & safety: B: Hazards to the environment (consider animals, plants, microorganisms, etc.) For humans, identify those persons who could be exposed to the hazard. See Note 2 GM03-11-2007 Form design M.Iosson 2007 Page 3 SCBS risk assessment form for project reference no: ................................ (Insert ref. number as allocated by SCBS) Likelihood of hazards associated with GMM being realised. A: Hazards to human health & safety (Take into account any disabling mutations in the GMM; the scale and type of operations, plus the containment level to be used. Also take into account any possible routes of transmission of the GMM to potential hosts.) Use the terms "High", "Medium", "Low" or "Negligible" and give B: Hazards to the environment details of scale and type of operations to be done with each GMM. Note also that the likelihood of environmental hazards being realised will depend on the nature of the “receiving environment”, i.e., the occurrence of potentially susceptible hosts in the neighbourhood of the containment facility Expand box as required See Note 3 Consequence of hazards being realised. (For the purpose of this assessment, assume that there are no barriers between the GMM and the environment, and that it can colonise or enter the environment.) Use the terms "Severe", "Medium", "Low" or "Negligible" and give details of the expected consequences GM03-11-2007 A: Human health & safety B: Environmental safety Form design M.Iosson 2007 Page 4 SCBS risk assessment form for project reference no: ................................ (Insert ref. number as allocated by SCBS) Estimation of risk magnitude A: Risks to Human health & safety (Magnitude of Risk = "likelihood" x "consequence" of causing harm.) Use one line for each GMM. B:Risks to the Environment See Note 4, "Risk matrix". For each GMM where the risk is not "effectively zero" at Containment level 1, please identify the nature of all the "non-zero" risks. See Notes 5 and 6. Identify all additional measures necessary to control the above risks. State class of activity (equivalent to that level of containment with characteristic measures identified as necessary in Q6) See Note 6 Class 1/Class 2/Class 3 (please delete as applicable) Note: Activities in Class 2 and/or Class 3 must be notified to HSE [Please delete part B if not applicable] Part B: Projects involving the use of GMOs that are not microorganisms. GM03-11-2007 Form design M.Iosson 2007 Page 5 SCBS risk assessment form for project reference no: ................................ (Insert ref. number as allocated by SCBS) Identify all potentially hazardous properties of the GMO(s) Take into account any toxic or allergenic effects of expression in the GMO; ability to transfer genes to, or interfere with other organisms in established ecosystems; acting as a novel reservoir for a pathogen, etc. See Note 2 on p. 1. Estimate the likelihood that these hazards could be realised. A: Hazards to human health & safety B: Hazards to the environment A: Hazards to human health & safety Take into account the containment measures to be applied. Use the terms "High", "Medium", "Low" or "Negligible" and give details of scale and type of operations to be done with each GMO. Estimate the consequence of these hazards being realised. B: Hazards to the environment A: Human health & safety (For the purpose of this assessment, assume that the GMO is not contained.) B: Environment Use the terms "Severe", "Medium", "Low" or "Negligible" and give details of the expected consequences GM03-11-2007 Form design M.Iosson 2007 Page 6 SCBS risk assessment form for project reference no: ................................ (Insert ref. number as allocated by SCBS) Estimation of risk magnitude A: Risks to human health & safety (Risk = "likelihood" x "consequence" of causing harm.) Use one line for each GMO. See Note 4, "Risk matrix" B: Risks to the environment For each GMO where the risk is not "effectively zero", identify the nature of these "non-zero" risks. A: Risks to human health & safety B: Risks to the environment Identify all measures necessary to control the above risks. A: Risks to human health & safety B: Risks to the environment GM03-11-2007 Form design M.Iosson 2007 Page 7 SCBS risk assessment form for project reference no: ................................ (Insert ref. number as allocated by SCBS) Based on the information in Q12, does the GMO pose any greater risks to human health and safety than the nonmodified parent? See Note 3 Yes/ No (please delete as necessary) (Note: the answer to this question is independent of any additional control measures identified above.) If "Yes" the project must be notified to HSE. Summary Statements The main sources of information for "lay" members of the Committee are the "Statement on overall risks" and the Lay Summary. They will be copied and submitted to the Committee for formal approval. Sufficient detail, in non-technical terms, should therefore be given to allow them to understand the aims of the project, and to confirm the low–risk nature of the project. Statement on the overall risks associated with this project Use the information provided in Parts A and/ or B to draft this statement. You should identify the most hazardous elements of the project, and any procedures necessary to control the associated risks. Extend the section as necessary. A: Risks to human health & safety B: Risks to the environment GM03-11-2007 Form design M.Iosson 2007 Page 8 SCBS risk assessment form for project reference no: ................................ (Insert ref. number as allocated by SCBS) Lay summary of the project Outline the aims and expected results of the project. Where possible, avoid the use of acronyms and abbreviations, and explain or spell out any used. ("DNA" and "RNA" need not be spelt out.) Clearly identify the risk class for the project. Describe the measures to be put in place to control the identified risks. Particular attention should be paid to those measures taken to decontaminate waste and / or equipment and/or to prevent dissemination of GMOs from the laboratory. Note that the Regulations now require that waste containing GMMs must be inactivated by validated means. [Expand box as required.] GM03-11-2007 Form design M.Iosson 2007 Page 9 SCBS risk assessment form for project reference no: ................................ (Insert ref. number as allocated by SCBS) Declaration and signature Please sign and date the declaration below before returning this form to : The Biological Safety Officer Safety Services Physics. If this form is used to assess the risks of a new project proposal, please contact the BSO for the allocation of a project reference number before submitting your proposals. I declare that the information given in this form summarises my intentions for of GM project number ………….., (Insert ref. number as allocated by SCBS) and represents my assessment of the risks associated with that project. Name………………………………………….(Please type) Area/ School………………………………………. Signed……………………………………… Dated GM03-11-2007 / /200… (insert date) Form design M.Iosson 2007 Page 10