Risk Assessment Guidance for Human Tissue

Guidance Notes for Human Tissue Risk Assessment Form
IMPORTANT - These guidance notes refer to completing a risk assessment form for risks to human tissue; additional generic forms from Occupational Safety, Health and
Environment Unit (OSHEU) need to be completed for risks to individuals, facilities and equipment.
1.
Prior to carrying out the risk assessment, write out the procedure in the form of a method statement or safe operating procedure or refer to an appropriate existing procedure [e.g. standard manual] to ensure that you have included and considered all of its components.
2.
Under the provisions of the Human Tissue Authority Licence Regulations risk assessments must be carried out for all practices and processes in relation to licensable activities
. This can initially be a simple consideration of the activity/procedure in order to identify potential hazards to human tissue. Should none be identified, no further action is required. If a potential hazard or hazards are detected, then you must carry out a full assessment using this form.
3.
Remember to consider all potential hazards from procurement to use and storage. Things to consider include package failure, delay or loss in transit, malfunction of storage facilities, unauthorised access to tissue samples, incorrect procedures being carried out, untrained personnel handling the tissue and any other hazards that result in tissue loss.
4.
The hierarchy of control measures employed should primarily be aimed at eliminating or reducing the hazards in a procedure, followed by early detection of risks and contingency planning. One example of a hierarchy of control measures is regular maintenance of storage facilities to prevent failure; followed by installation of an early warning system to detect changes in storage temperature; and finally alternative on-site storage facilities in the event of storage failure.
Other examples of control measures include appropriate training, written procedures [Standard Operating Procedures, Method Statement] and supervision by an appropriate person. The level of supervision must always be appropriate to the competence of the individuals involved in the work activity.
Any special training required to ensure that persons involved in the work activity can operate safely should be detailed. This is particularly important so that persons can understand and comply effectively with a Standard Operating
Procedure (SOP), Scheme of Work, or Method Statement, where this has been formulated.
You should record when each control measure has been implemented and the new numerical risk rating following its implementation. The aim should be to achieve a progressive reduction down to a final risk rating of 6 or less.
5.
Risk should be calculated for an existing activity taking into account any control measures in place. This is estimated by considering both the likelihood of exposure to a risk and the severity of the consequences of such an exposure.
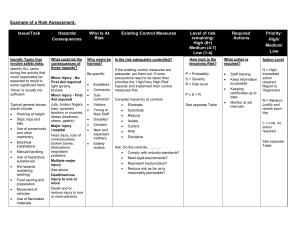
The calculation of risk should be done as follows:
Page 1 of 3
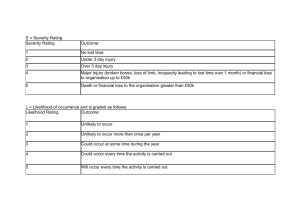
Select an appropriate number for both Likelihood and Severity from the bottom of the table and multiply them together. Cross reference your score on the coloured part of the table and this is your risk rating.
Likelihood
5
Almost
Certain
5 10 15 20 25
4 Very Likely
3 Likely
4
3
8
6
12
9
16
12
20
15
2 Unlikely
1
Very
Unlikely
2
1
4
2
6
3
8
4
10
5
Severity
No tissue damage/loss
1
Minor tissue damage/loss
2
Significant tissue damage/loss
3
Tissue destroyed but replaceable
4
Tissue destroyed and irreplaceable
5
Score Action to be taken:
0-5 Low Risk: No further action needed.
6-9 Medium Risk : Appropriate additional control measures should be implemented
10-25 High Risk: Work should not be started or should cease until appropriate additional control measures are implemented.
6.
Include all tissue potentially at risk.
7.
Contingency planning is required to limit the extent of the risk arising from an adverse event and for regaining control of the area as quickly as possible.
8.
Appropriate arrangements for monitoring the efficacy and continued employment of the control measures must be put in place. This may include regular inspections and maintenance checks, and reviewing documentation and training needs.
9.
A copy of the risk assessment must be given to every individual carrying out the activity and each copy must be countersigned by the holder. Copies of the risk assessment must be sent to the relevant School/Unit PD and held on site by the
PI.
10.
The risk assessment must be reviewed at regular intervals. There is no specified interval in Safety Law, but, we recommend the following simple guide depending on the estimated level of risk:
0-5 – every 3 years
6-10 – every 2 years
12-25 – every year
You must also review the assessment whenever there has been a significant change in the procedure or work circumstances or there is any reason to suspect that the original assessment is no longer valid. Examples would include new
Page 2 of 3
information on the hazard indicating a higher or lower level of risk, personnel changes, changes in equipment or substances used, change of location, following an accident or incident.
.
WARNING!
Completion of the assessment is not an end in itself, merely the beginning.
You must consider it as an active document for frequent reference, particularly when preparing associated method statements, safe operating procedures and training .
Page 3 of 3