The Origin of Diversity (within and across Species)
advertisement

A Unified Theory of Biodiversity
A Role for Differential Susceptibility to Disease in the Origin and
Stabilization of Biodiversity: A Unification of Twin Problems in Ecology
and Evolution
Alex Bäcker1,2 and Ulrik R. Beierholm2
1: Sandia National Laboratories, P.O. Box 5800, Albuquerque, NM 87185
2: California Institute of Technology, MC 139-74, Pasadena, CA 91125
Correspondence or request for materials should be addressed to
alex@caltech.edu and beierh@caltech.edu .
One of the central goals of ecology is to identify mechanisms that
maintain biodiversity (Kerr et al., 2002; Chesson, 2000). The stability of
biodiversity over millions of years of evolution has been one of the most
persistent puzzles of ecology and evolution {Hutchinson, 1961
#723}{Wilson, 1992 #722}. This problem has two separate incarnations
that, albeit traditionally treated in different literatures, share
fundamental features: species coexistence and genetic polymorphisms.
We postulate that these two problems are instances of one general
problem and present a model that can explain both puzzles.
Introduction
Why are there so many species? Why haven’t the fittest of them all out
competed the rest to extinction? And, intimately related and yet not
recognized as such in the vast majority of the literature, why are close to
30% of gene loci in most every species examined polymorphic? Why
haven’t the fittest of all genes become fixated? Modern textbooks present
these enigmatic facts with no accepted explanation for them (Futuyma, ;
Strickberger). Furthermore, the biochemical causes that sustain differences
in polymorphisms between genes are unknown (Strickberger, 2000).
Darwin’s theory of natural selection requires resources to be limited by the
abundance of individuals in such a way that the gain of one genotype implies
necessarily the loss of another; it is frequencies, or fractional abundances,
that matter in Darwinian evolution. As Darwin himself pointed out in The
Origin of Species, twenty species coexist in a lawn which is mowed
regularly, while only eleven persist in one given free rein. At the time,
Darwin was attempting to explain the disappearance of nine species after
mowing was interrupted. One hundred and fifty years later, with Darwinian
evolution ingrained into our intuition, what is puzzling is quite the opposite:
the fact that eleven species survived.
Darwinian theory sustains that, eventually, only the fittest among
competitors will survive. Faced with the astounding diversity of genotypes
both within species and across them, evolutionary biologists developed
neutral theory, which argues that most mutations are neutral, and are thus
not acted upon by natural selection. Darwin, they argued, was not wrong; his
theory is simply excluded from playing a part in the fate of most mutations.
Every study of human genetic diversity since Cavalli-Sforza and Edwards in
1964 has assumed that genetic polymorphisms are all neutral (Wells, 2002,
p. 23).
And yet the evidence suggests that most accumulated mutations are not in
random directions, as would be expected by the neutral theory (PNAS
2002?). Note that this does not imply that they are adaptive in any particular
sense, but could derive from other constraints, such as a constant push away
from a competing species. As Abrams put it (2001), “decades of experiments
studying hundreds of species pairs have identified no conclusive cases of
competitive equivalence”. Furthermore, contrary to the predictions of neutral
theory, the most polymorphic genes, such as the HLA alleles, are at loci
under great selective pressure due to their crucial role in resistance to
disease.
Likewise across species, Darwinian theory states that a single resource
should only create a niche large enough for a single species to prevail, yet
several studies have in systems seemingly defined by small number of
ressources found large number of species coexisting, a paradox that in the
marine biological litterature is known as the “plankton paradox”.
Theorists have proposed explanations for coexistence by resorting to
temporal or spatial inhomogeneities (e.g., Stewart and Levin 1973, Kondoh
2003) or nonequilibrium coexistence (e.g., Koch 1974; Armstrong and
McGehee 1976a, b; Huisman and Weissing, 1999), or, for the special case of
two coexisting species, by differential fitness ranks in juveniles and adults
(McCann, 1998).
Regulation of populations by density-dependent mechanisms is one of the
basic tenets of theory in population biology. Yet, most density-dependent
mechanisms affect all species in a given niche, and thus do not solve the
coexistence conundrum. We show here that the critical requirement for
coexistence is differential vulnerabilities to density-dependent mechanisms
and we argue that contagious disease is likely to be a major contributor to
such differential density-dependent mechanisms
In this article we present a unifying framework to explain biodiversity at
various levels, from the unexplained pervasiveness of polymorphisms to the
coexistence of species in an ecological niche. Second, we show that
coexistence of any number of species can result in stable equilibrium
without the need for spatial or temporal inhomogeneities. Third, we show
that this mechanism is robust to fluctuations in resource availability. Fourth,
we show that shared vulnerabilities shared between species or genotypes
lead to competitive exclusion, while a unique vulnerability in each species
leads to coexistence.
Results
The probability that multiple species will de novo have identical
fitness is very low. For a stable equilibrium to exist with multiple coexisting
genotypes, what is needed is a restorative force that reduces the ratio of
mortality to birth rates when a population’s size fluctuates downward, and
vice-versa. More precisely, what we seek is that the effect of each species on
its own growth-rate be more inhibiting1 than that of it on all other species
(Cheeson, 2000). What , we ask, is this species-specific population-sizedependent force likely to be?
Any mechanism that increases fitness (fast enough) as population size
decreases will work. Predators that specialize increasingly in a prey as its
frequency increases decrease its fitness by the same mechanism of
differentiating the vulnerabilities, but this is a slow effect if a change in
behavior has to evolve. If the predators are shared, with equal
1
The term intra-specific and inter-specific competition is commonly used in the literature, but we prefer the
term inhibition, for the interaction need not be competitive: in our case, as we will show, it represents the
fact that it is easier to catch a disease from a con-species than across species.
vulnerabilities, the mechanism will not produce coexistence. Indeed, both
field studies and theoretical studies show differences in predation selectivity
for different prey species, if they exist at all, often cannot account for
coexistence (Harding, 1997; GAEDKE and EBENHOH, 1991, Spiller and
Schoener, 1998; but see Martínez et al., 1993; Sundell et al., 2003). In
addition, the incidence of predation as a mortality cause has probably been
overestimated because sick animals are more likely to be predated on, and
signs of predation are more easily detected than those of infection.
Moreover, differential susceptibility to predation is unlikely to account for
the massive coexistence of polymorphisms within species.
Disease, in contrast: 1.Has an instantaneous effect on fitness as a function of
density changes; 2. Evolves very fast, because disease-bearing agents are
usually small parasites with short generation times.
How common is predation vs. disease as agent of death throughout the
different forms of life? Although more experimental studies on the matter
are called for, there is strong experimental evidence for density-dependent
infection and for disease mediating much of the strong density dependence
observed in an aquatic insect (Kohler and Hoiland, 2001).
The idea that an arms race versus parasites drives evolution of sex-related
genes has recently received support (Haag et al, 2002). An often ignored
consequence of the Red Queen hypothesis for the origin of sex, a theory
which has received considerable empirical support in the last few years, is
that, if correct, more than half of all deaths (or losses of fertility) in all
sexual species (or their ancestors) must be caused by parasites. This
suggests parasites as a natural candidate for the force determining population
sizes at equilibrium.
A fundamental requirement of stability in the theory is that parasites not
spread across the two coexisting populations (or that the interspecific effect
be less than the intraspecific), for if they do, the effective population size
determining the likelihood of an individual becoming infected will be the
combined total across both populations, and thus a reduction in numbers of
one of the two will not lead to a corresponding replenishment, leading to an
eventual extinction of the population with lower fitness. This leads to a
fundamental prediction of the theory: species which share all their
vulnerabilities will not coexist in the long-term.
Indeed, the role of endemic infection in host population dynamics is a major
open problem (Begon et al., 1998).
For drowning sailors at sea, survival is not a competition between sailors; it
is a battle against the sea. Analogously, species in today’s populated Earth
live in a sea of parasites. we suggest Darwinian competition is not the major
factor in the evolution of diversity on Earth. Instead, successful defense from
parasites is.
As a consequence, it seems plausible that in recent evolution, more of our
genes have evolved to combat parasites than have evolved to adapt to our
physical environment. This is consistent with the surprising results of a
recent study of [XXX species (Japanese group)] found that only [XXX]
genes are required for the function of [XXX] in an isolated environment, a
point that was not emphasized by the authors (was it?). Indeed, Flor found
27 genes in the flax plant, Linum usitatissium, that confer resistance against
a single fungal rust pathogen, while the pathogen had a similar number of
genes allowing it to overcome resistance conferred by those host genes
(Flor, 1956, cited in Strickberger, 2000, p. 575).
We hypothesize that a principal cause of sequence polymorphisms, not only
in the MHC, but throughout genomes, are frequency-dependent mechanisms
related to pathogen resistance. Unlike previous theories of frequencydependent mechanisms for the MHC (Hedrick, 2002 and references therein),
ours does not call for temporal variation in selection coefficients. There is
indeed experimental evidence for frequency-dependent selection of alleles,
although the mechanism behind this had remained a puzzle (Kojima and
Yarbrough, 1967). I further suggest that loci not associated with disease
resistance that share susceptibility to parasitic infection by parasites
coexisting with the host will tend toward a single neutral cloud in sequence
space, through the extinction of all other genotypes. In contrast, species
dissimilar enough not to share vulnerabilities to parasites can coexist in a
stable equilibrium.
There is indeed recent experimental evidence that predators exert frequencydependent selection on prey (Bond and Kamil, 2002).
Species are traditionally defined by the existence of gene flow within species
and not across them. This definition does not easily extend to clonallyreproducing “species”, and yet clonal organisms are found to cluster in
genetic space just as sexually-reproducing species do. We suggest that
parasites are responsible for such clustering. This suggests a new functional
definition of species that applies to clonally-reproducing creatures, one
based on common susceptibility to parasitic infection. If the diversity of
populations is indeed limited by the exclusion of genotypes with common
susceptibility to parasites, organisms with no parasites, such as viruses,
should exhibit the greatest diversity of all living organisms. Indeed, viruses
such as HIV and the influenza viruses confirm this prediction: the variability
of individuals within a viral population is not limited to a few peaks within a
fitness landscape (of course, some limitations in genotype composition
allowed are given by the adaptive landscape: an HIV virus incapable of
replicating, for example, will not propagate in any population).
Note that this does not necessarily lead to massive parasite-driven
extinctions, in the sense of an entire lineage lost with no closely related
genotypes surviving, because the very mechanisms described above ensure
that the populations in ways of extinction have close relatives that are
susceptible to the same parasites. In this way, parasites ensure their longterm survival. (But Van Valen 1973 extinction power law suggests that
p(extinction) constant through time?)
Model
To illustrate these ideas we use a common logistic population model with a
monod type growth rate (ref monod) and added the concept of disease to be
simulated (see Methods). For simplicity the model assumes species feeding
off abiotic resources as in models of plankton populations, but the model can
easily be expanded to species feeding off biotic resources (predator/prey
models) with qualitatively similar results (see supplemental material).
Two versions of this model was used, one to examine the importance of
disease for the interaction between species, and a slightly more complex
model examining the importance of disease for different genotypes within a
single species.
A number of species (6 in experiment 1, 2 in experiment 2) is simulated
feeding off a number of resources less than the number of species (3 in
experiment 1, 1 in experiment 2). The rate of infection was a function of the
density of infected, a typical assumption in similar models (ref.).
In experiment one, six species were injected into a system with a slow
inflow of three resources. Figure 1a shows how the population density of
each species varies over time for the case without any diseases. The most fit
species out competes the rest within ~10 generations.
Figure 1b shows an example of a simulation of a system with six species
feeding of 3 resources with diseases incapable of spreading across species.
Each species is therefore regulated by its own disease. After 20-30 days the
system reaches equilibrium with all species surviving despite having fewer
resources than number of species. The diseases regulate each species
keeping each from growing to a density where it would out compete the
other species.
Figure 1c shows a similar system for which the mortality rate for diseased is
higher, leading to damped oscillations of the density distribution. As the
diseases spreads through the population, the population density is lowered to
a level lower than what can sustain the disease, until the disease has almost
died out, allowing the species density to grow again and the cycle repeats.
To further examine the relationships in the model we ran 100 simulations for
several combinations of the infection rate, alpha, and the mortality rate of
the infected, beta. In figure 2a we plot the average number of species
surviving in these systems, with black being 1 survivor and white being all 6
species surviving. .
For low values of beta, the disease permeates the species in the system but
does not significantly influence their fitness, and the system therefore acts as
a system without disease. As an analogy, the disease can be thought of as a
simple cold, that may spread easily but which seldom has lethal
consequences.
For intermediate values of beta the disease exists in equilibrium in the
population and has a large enough effect on a species fitness, so as to lower
it enough for other species to be able to compete with the infected species.
As beta increases, the disease gets so lethal that it tends to kill the carriers of
the disease before they have a chance to spread the disease to uninfected.
Notice that this cutoff is dependant on the rate of infection, alpha. When
beta>alpha the disease will be so deadly that it will be automatically
eradicated from the population (can be seen mathematically by solving the
equations for equilibrium by requiring dI/dt=0 and dN/dt=0).
We can try examining the same question in a system with cross species
infection (see methods). Each species can now be infected by any other
species, eliminating the species specific frequency dependant regulation,
which allowed the coexistence of more species than resources in the system.
When numerically simulated we find no systems with coexistence for any
alpha and beta combination (figure 2b).
These simple simulations show that diseases can function as a density
dependent population regulator assuming the disease is specific enough to
only target single species and can therefore promote coexistence of several
species.
In order to study how diseases can promote genetic polymorphism we
included sexual reproduction in the second experiment. To do this we
expanded our model to simulate two diploid species, each having three
genotypes aa, ab and bb available in the population. We assumed that the aa
genotype was advantageous and gave a 15% higher growth rate, the ab gave
5% higher growth rate. Furthermore for this experiment we assumed that the
available resources were biotic, and that the inflow to the system was
therefore a function of the current amount of resources.*
Two species were introduced in the system, with species 1 in addition
having a 15% higher growth rate than species 2. Without the influence of
diseases we would expect the aa genotype of species 1 to out compete the
other types, as is what we see in figure 3a.
The introduction of a non-specific disease (fig. 3b), able to spread across
genotypes and species, causes a depression of the entire population, but does
not stop species 1 from out competing everything else.
However, if the disease is specific enough to only spread within a species
(fig. 3c), we see that species 1 is sufficiently affected by the disease to allow
the genotype aa of species 2 to coexist. This is exactly the same as what we
saw in the first experiment.
If we further more assume that each genotype renders immunity to different
diseases, essentially leaving each genotype with its own disease, we find
complete coexistence between all species and genotypes (fig. 3d).
More complicated intermediate systems are of course also possible, leading
to either exclusion or coexistence dependent on the parameter values.
If a gene confers an immunity to a disease, that constitutes an advantage
which can allow it to survive in a population despite giving a seemingly
lower growth/reproduction rate. This shows that the same mechanism that
allows coexistence of species can also explain why there is an advantage in
having multiple versions of a gene in a population if it confers immunity
towards a certain disease. Through the specificity of diseases, specific
species as well as genotypes can be down regulated allowing for the survival
of species and genotypes with a lower fitness. This also shows how
experimenting on cultures in isolation can yield very different results than
doing experiments under more ‘natural’ conditions.
Evidence
Available evidence suggests that introduced competitors can drastically
reduce the abundance and distribution of native species, but rarely lead to
complete extinction, at least in terrestrial continental habitats (Frankel and
Soulé 1981, Soulé 1983 as cited in Diamond, 1984). The reason pointed out
by Soulé is that the relative competitive ability of species generally varies
over habitats, and that it is probably rare for a species to be superior to
another in all of its habitats. An alternative explanation would be that species
show a reduced vulnerability to contagious disease once their population has
been decimated.
Indeed, many parasites have been found living on plankton (Fahmi and
Hussain, 2003). For example, not one but several viruses that are pathogenic
to the common marine prasinophyte Micromonas pusilla (a small flagellate)
have been isolated from sea water in several locations (Fuhrman, 1999). The
recent realization that viruses may be the most abundant organisms in
natural waters, surpassing the number of bacteria by an order of magnitude,
has inspired a resurgence of interest in viruses in the aquatic environment.
New methods have yielded data showing that viral infection can have a
significant impact on bacteria and unicellular algae populations and
supporting the hypothesis that viruses play a significant role in microbial
food webs. Novel applications of molecular genetic techniques have
provided good evidence that viral infection can significantly influence the
composition and diversity of aquatic microbial communities (Wommack and
Colwell, 2000). Field cage studies have shown that nematode parasites can
reduce competitive superiority of the dominant species in a Drosophila
community (Jaenike, 1995; Gillis and Hardy, 1997), and trematodes can
favor invasion by a competitor in mussel communities (Calvo-Ugarteburu
and McQuaid, 1997). Consistent with the predictions of our theory, plankton
parasites can be so unique so as to allow the identification of the host
through the parasite (Noble, 1972).
The importance of viruses for plankton diversity were put in question by
results of both field and mesocosm studies that suggest that only ca. 20% or
less of bacterioplankton and phytoplankton mortality is attributable to viral
infection (Wommack and Colwell, 2000). The present study shows that a
modest parasite-induced mortality in equilibrium is consistent nevertheless
with a role of parasites in maintaining biodiversity.
These findings may also explain a seemingly unrelated puzzle, that of why
different species of the D. willistoni group, as well as E. coli clones from the
intestinal tracts of animals as diverse as lizards and humans from different
continents share a narrow distribution of allozymes (enzyme
polymorphisms) (Strickberger, 2000). If equilibrium distributions of
polymorphisms were determined by minute fitness differences between
alleles given by their environment, as proposed by Kojima and Yarbrough
(1965) in the explanation given in today’s evolution textbooks (Strickberger,
2000), it would be hard to account for these distributions being equal in
environments as varied as those of lizard and human guts. The similarities
may be easier to account for if, on the other hand, the distributions are given
by the steady-state frequencies for viral parasites, which depend more on
their host than on the environment, and as such are more likely to remain
constant for the same host in diverse environments.
An intriguing example of this exists between the annual
legume Chamaecrista fasciculata (Caesalpiniaceae) and the large, dominant
perennial grass Andropogon gerardii (Poaceae), which coexist in the Kansas
tallgrass prairies. Holah & Alexander (1999) found that both species grew
more poorly in soil from the root zone of Chamaecrista than in Andropogon
soil, and that the effect was associated with fungi found uniquely on
Chamaecrista roots. Pathogenic fungi “cultured” on Chamaecrista roots
shift the competitive outcome against the dominant perennial grass,
facilitating coexistence of the two plant species.
Understanding the host ranges of pathogens within a local plant assemblage
and the possible adaptation by plants to actively culture pathogens that
increase their competitive ability is a largely unexplored, but potentially
fruitful field (Gilbert, 2002).
Indeed, for plants, native herbivores ( Janzen 1970; Hulme 1996) and
disease ( Augspurger 1988; Harper 1990; Alexander 1992) have been
hypothesized to contribute to the maintenance of plant species diversity, for
which the density of seeds and the probability that a seed will mature are a
function of the distance from the parent tree. This mechanism has been
regarded as “far from an easy solution” (Chesson, 2000). We show here that
a simple model that applies for any living being, and not plants alone, can
lead to coexistence of any number of species.
Competitive exclusion has been formulated as a restriction in equilibrium to
one species per resource. An equivalent principle exists for predation that
says that ???????Remove?
Jerome and Ford (2002) recently provided evidence both for parasitic
specialization for similar hosts, and found that the number of parasitic races
in a natural environment was slightly greater than the number of host
species.
Brunet and Mundt (2000) found frequency-dependent selection in wheat in
the presence of disease, but not enough to maintain polymorphisms. They
used only 3 races of a single pathogen, though, which our model predicts
would be insufficient to maintain the 4 genotypes they used in their
experiments. Furthermore, while the genotypes they use show different
susceptibilities to the pathogens used, it is not clear whether they show
differential contagion of the pathogens, an essential requirement for
coexistence. Realistic natural ecological conditions would have many more
pathogens, and our model suggests this is essential for the maintenance of
polymorphisms; future experiments should take this into account.
Discussion
We have seen that parasites can promote diversification, as can predators.
Given the apparent simplicity of the former compared to the latter, could the
Cambrian Explosion be due then, not to the appearance of metazoans
(multicellular animals) as theorized by Stanley exactly 30 years ago, but
rather to the appearance of the first parasites? And if so, what was the
critical evolutionary advance that made them possible? After all, viruses are
simpler than bacteria in terms of the size of their genome. Could it be that,
once again (after the shock of the small number of genes in the human
genome), evolution suggests that size isn’t everything? We know, for
example, that the genetic content of viruses (e.g. Giorgi et al., 1983) and
mitochondria (e.g. Leblanc et al., 1995) –which have become the ultimate
parasites-- is extremely optimized, to the point that, in addition to having
highly compact genomes, they have both evolved overlapping genes (see
Normark et al., 1983 for a review). Thus, small genomes may be an
evolutionary endpoint that follows, rather than precedes, large genomes.
And the reason why the metaphytes (plants) and metazoans (multicellular
animals) mysteriously originated almost simultaneously (Schopf et al., 1973)
may be not that the origin of metazoans brought about the diversification of
the metaphytes, as proposed by Stanley (1973), but rather that the critical
innovation necessary for both was that of parasites and endosymbiosis,
which allowed the appearance of mitochondria and chloroplasts and, through
the cropping principle (Paine, 1966), caused the diversification of life forms
we know as the Cambrian explosion.
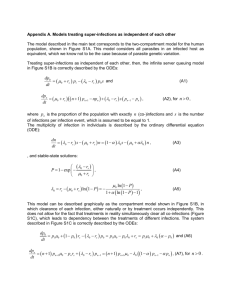
Methods
For all simulations in this paper we use a modified version of a Monod
model (Monod, 1950), assuming that n number of species living on p
number of resources are governed by a set of differential equations. The
abundance of species i, Ni, is given as
dN i
N i ( i mi ) ( i mi ) I i
dt
where μi is the growth rate of species i and mi is the mortality rate of the
non-infected subpopulation of species i.
The second term implies that we have introduced a subset of Ni, Ii, which
have been infected by a disease which, assuming βi>mi, increases the
mortality rate of the infected.
βi is the mortality rate of the infected subpopulation and the abundance of
infected within species i is given by either
dI i
i ( Ni I i ) I i i I i
dt
or
dI i
i ( Ni I i ) I j i I i .
dt
j
αi is the rate of infection per non-infected. The difference between the two
equations for Ii determine whether diseases can spread across species or are
confined within a species.
The growth rate, μi , for species i is given by a minimum function over all
the available resources,
rR
ri R1
rR
, i 2 ,.... i p
R p K ip
R1 K i1 R2 K i 2
i min
where Kij is the half-saturation constant for species i with regard to resource
j and r is the maximal growth rate, in our simulations set to 1.
The abundance of resource j, Rj, in the system is described by
dR j
dt
D( S j R j ) cij N i i .
i
D is the inflow rate, Sj is the maximal availability of resource j and cij is the
uptake of resource j that the growth of species i causes.
Notice that if we set the infection rate to zero, αi=0, and the mortality rate of
the infected sub-population equal to that of the non-infected, βi=mi, we
retain the formulas for a traditional Monod model.
In all simulations, Kij and cij were chosen as random variables uniformly
distributed between [0.2:1.2] and [0.02:0.12], r=1, D-1=mi-1=4 days, Sj=10.
The infection rate, αi, varied between 0.002 and 1.024 pr non-infected, and
the infected mortality rate, βi, was between 0.3 and 6.65. Both αi and βi are
given in the relevant figures.
Sexually reproducing model:
Assume two species which each has two genotypes in diploid organisms.
This allows for 3 possible combinations in each species: aa, ab and bb.
To introduce competition, we will assume that genotype aa has a 15% higher
growth rate and ab 5% higher growth rate relative to bb.
Species 1 will also be assumed to have a 10% higher growth rate than
species 2.
Abundance of each species i, and each species genotypes j, were calculated
as above except that each genotype was tracked
The total species (Uninfected plus infected):
dNaa,i/dt=(Naa,i*(pNab,i/2+pNab,i)+Nab,i.*pNab,i).*μ.*1.15 Naa,i*m–
ΣdIaa,i,d(βaai-m)
dNbb,i/dt=(Nbb,i*(pNab,i/2+pNbb,i)+Nab,i*pNab,i).* μ *1.00 Nbb,i*mΣd Ibb,i,d(βbbi-m)
dNab,i/dt=Nab,i*(pNab,i/2+pNaa,i/2+pNbb,i/2)+Naa,i*pNbb,i)* μ*1.05- Nbb,i*mΣd Iab,i,d( βabi-m)
βji is the mortality rate of species i, genotype j, assumed to be equal across
diseases. m is the mortality rate of the uninfected, μ is the growth rate of
species i.
pNab,i is the normalized abundance of species i, genotype ab, that is pNab,i =
Nab,i /Σj Nji
Infected abundance:
dIji,d/dt=(Nji – Σd Ij,i,d )* Σf Σg (αijfgd*Nfgd ) - Ij,i,d* βji ;
where j is either aa, bb or ab and d is one of the diseases.
αijfgd represents the probability of species i, genotype j, of getting infected
by species f, genotype g by disease d.
The equations for both models were solved numerically using a stochastic
second-order Runge-Kutta algorithm, programmed in C++.
Acknowledgements:
This work was supported by an award to A.B. from the DOE Office of
Science’s MICS Program at Sandia National Laboratories and by the
Beckman Institute at Caltech. Sandia is a multiprogram laboratory operated
by Sandia Corporation, a Lockheed Martin Company, for the United States
Department of Energy.
References:
1. Abrams, PA (2001). A world without competition. Nature 412, 858 –
859.
2. Alexander, H.M. (1992) Fungal pathogens and the structure of plant
populations and communities. The Fungal Community, its
Organization and Role in the Ecosystem (eds G.C. Carroll & D.T.
Wicklow), pp. 481 497. Marcel Dekker Inc., New York, NY.
3. Augspurger, C.K. (1988) Impacts of pathogens on natural plant
populations. Plant Population Ecology(eds A.J. Davy, M.J. Hutchings
& A.R. Watkinson), pp. 413 434 Symposium 28 of the British
Ecological Society, Blackwell Science, Oxford.
4. G. Calvo-Ugarteburu and C.D. McQuaid, Parasitism and introduced
species: epidemiology of trematodes in the intertidal mussels Perna
perna and Mytilus galloprovincialis. J. Exp. Mar. Biol. Ecol. 120
(1997), pp. 47–65.
5. Harper, J.L. (1990) Pests, pathogens, and plant communities: an
introduction. Pests, Pathogens and Plant Communities (eds J.J.
Burdon & S.R. Leather), pp. 3 14. Blackwell Scientific Publications,
Oxford, UK.
6. Holah JC, and Alexander HM. 1999. Soil pathogenic fungi have the
potential to affect the co-existence of two tallgrass prairie species. J.
Ecol. 87:598-608
7. Janzen, D.H. (1970) Herbivores and the number of tree species in
tropical forests. The American Naturalist, 104, 501 528.
8. Strickberger, MW (2000). Evolution. Jones and Bartlett, Sudbury,
MA.
9. Wells, S (2002). The Journey of Man. A Genetic Odissey. Princeton
University Press, Princeton, NJ.
10.Monod, J. La technique de culture continue, theorie et applications.
Ann. Inst. Pasteur (Paris) 79:390-410, 1950
Figure Legends:
Figure 1
Population density as a function of time for six species living off 3
resources. a) A system with no within species or across species diseases.
b) System with an introduced disease with no infection between species and
with diseases with low infected mortality rate beta = 0.65 or high mortality
rate of infected, beta = 3.45 (c).
d) Same system as in c) but with across species contamination.
Figure 2
Plot of average number of survivors given infection rate alpha and mortality
rate beta. 1000 random systems were generated, each with 6 species living
off 3 resources. The color coding shows the average number of surviving
species after 10,000 days(?).
In a) there is cross contamination between species (gamma=0.1?), in b) there
is no cross contamination (gamma =0).
Figure 3
Population density of two diploid species, with two different alleles for
which one homozygote has a higher fitness ie. higher growth rate, than the
other and the heterozygote has an intermediate value. A disease is included
in the system. In a) the disease can spread across species and across genetic
variation. In b) the disease is only able to spread within a species creating
survival of both species. In c) the disease is confined to spread within the
same species and only to species with the same alleles.
Figure 1
a
b
c
d
Figure 2
a)
b)
Figure 3
Include?:
As the differential vulnerability to virus and worm attacks of different
operating system attests to, our findings have implications for the design of
computer systems robust to attacks. They also suggest a method to engineer
stable biological (e.g. microbial) communities through the introduction of
one parasite per competitor.
Predicting equilibrium levels from vulnerabilities?
Indeed, susceptibility of different species to predation varies significantly
among related species, and predicts population declines (Jackson and Green,
2000).
Figure 1 shows that as long as the rate of infection is large enough, so that
the cost of dense populations is large enough to halt population growth,
coexistence will ensue, if the mortality rate of those infected is high enough
to halt population growth but low enough to allow effective transmission of
the disease. The minimum mortality rate for coexistence is constant across
infection rates, and relates to the birth rate: the mortality rate of infected
individuals has to be at least as large as the birth rate if a population is to
achieve a stable population size. In contrast, the maximum mortality rate for
coexistence shows a trade-off with contagion rates: the higher the rate of
infection, the more deadly a disease can be while still allowing coexistence,
because faster transmission allows contagion even if time to death is shorter,
and conversely, the higher the mortality rate, the higher the minimum
contagion rates necessary to ensure spreading of the disease.
At low transmission rates, oscillations in species abundance and infected
fractions are seen due to the lag between changes in population density and
the changes in infected fractions that follow. At high transmission rates,
these effects happen much faster, and the oscillations disappear.
(from introduction)
In the presence of multiple resources, stable equilibria with more than one
species, namely one per resource, exists. [ refs?] However when several
species forage off one common resource, traditional population biology
states that the more fit of the two should out compete the less fortunate one.
A persistent puzzle in evolution, one which has come to be known as the
“paradox of the plankton”, is the empirical fact that multiple species can
coexist per resource, contrary to the predictions of Darwinian doctrine. (ref?)
The idea that addition of a trophic level to a given food web tends to
promote increased diversity at the next lower trophic level was first put forth
by Paine (1966, cited in Stanley, 1973). In fact, this principle has been
invoked to explain the suddenness of the Cambrian explosion by Stanley
(1973), who hypothesized that the appearance of heterotrophic multicellular
organisms gave rise to the diversity of life that ensued during the early
Cambrian. Previous attention on the influence of predators and disease on
coexistence has centered mostly on shared predators (e.g. Abrams, 1999)
and diseases (Holt and Pickering, 1985; Anderson and May, 1986; Begon et
al., 1992; Dobson and Crawley, 1994; Abrams and Kawecki, 1999), and thus
on their negative effect on biodiversity. Indeed, shared susceptibilities lead
to competitive exclusion in our model. A model for the role of disease has
been presented for the limited case of two species and a single intra-species
disease (Venturino, 2001). In contrast, little work has been focused on the
effect of multiple infectious diseases on the coexistence of multiple host
species. Recent work suggests that multiple pathogens must be studied in
combination to understand ecological processes (Rohani et al., 2003). A
recent review on solutions to the paradox of the plankton (Scheffer et al.,
2003) made the implicit assumption that non-equilibrium is needed to solve
the paradox.
KEYWORDS: pathogens – parasites – contagious disease – coexistence –
maintenance of polymorphisms – evolution – ecology – frequencydependent selection – density-dependent mechanisms – ecological niche