Tarenna asiatica MS
advertisement

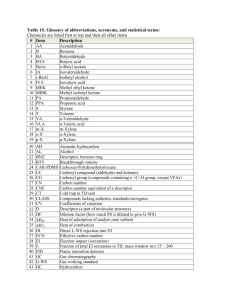
Phytochemical investigation of the gum resin of Tarenna asiatica Authors from our side: Sujatha P., Nagamani P., Ramabharathi V., Suryanarayana L., Appa Rao AVN. Abstract: Phytochemical investigation of Tarenna asiatica led to the isolation of ______ flavones and ______ terpenoids. Keywords: Tarenna asiatica, Rubiaceae, gum resin, flavonoids, terpenoids. Introduction: The genus Tarenna Gaertn. has about 370 species in tropical and subtropical Africa, Asia, Madagascar and Pacific islands [1]. Tarenna asiatica (L.) Kuntze ex K. Shum, Rubiaceae, is a medium sized tree with white flowers that grows in the southern states of India. The leaves of the plant are claimed to be useful in skin diseases [2]. A gum resin oozes out the leaf buds of these plants. The flavonoid corymbosin was isolated from the leaves of Webera corymbosa which is a synonym of T. asiatica [3]. D-mannitol was reported from the roots of T. asiatica [4]. Geniposidic acid, tarennoside and ixoside were reported from the methanolic extract of the leaves and twigs of Tarenna kotoensis (Hayata) Masam which is equivalent to T. asiatica (L.) Kuntze ex K. Schum. [5, 6]. Iridoid glycosides [7], mixed lignans-neolignans, chalcones [8] and lignan glucosides [9] were reported from (whole plant of) T. attenuata. Cycloartane glycosides were reported from the leaves of T. gracilipes [10-12]. Sesquiterpenes were reported from the stem of T. madagascarensis [13]. Literature survey revealed that the gum resin oozing out from the leaf buds of T. asiatica was not subjected to phytochemical investigation. Hence it was taken up. This is the first report on the phytochemical investigations of this gum resin. Experimental: Gum Resin: Gum resin oozing out of the leaf buds of T. asiatica was collected from trees growing in Narsampet, Warangal district, Andhra Pradesh, India in January 2012. Voucher specimen is being maintained in the herbarium of University college of Pharmaceutical Sciences, Kakatiya University, Warangal. Extraction: 25 g of the gum resin was extracted with benzene (50 mL X 2) in a conical flask and filtered. The filtrate was concentrated in a rotary evaporator to give the benzene extract which was dried in a desiccator (17 g). Isolation: 17 g of the benzene extract of T. asiatica was chromatographed on siliga gel using benzene, and benzene: acetone mixtures. Finally the column was washed with methanol. Benzene:acetone (90:10) eluates on concentration gave 2.5 g of a material. This was rechromatographed on RP (C-18) silica gel using acetonitrile:water (50:50) to give Ta-1 (110 mg) and Ta-2 (70 mg). Benzene:acetone (85:15) eluates on concentration gave 3.1 g of a material. This on rechromatography on normal phase silica gel and elution with benzene:acetone (94:6) gave Ta-3 (80 mg) and Ta-4 (38 mg). Benzene:acetone (80:20) eluates on concentration gave 3.3 g of a material. Rechromatography of this on RP (C-18) silica gel using methanol:water (85:15) gave TAC-6 (1.16 g). Benzene:acetone (70:30) eluates on concentration gave a material which on rechromatography on RP (C-18) silica gel using acetonitrile:water (60:40) gave TAC-7 (49 mg). Benzene: acetone (60:40) eluates on concentration gave 8 g of a material. This on rechromatography on RP (C-18) silica gel and elution with methanol:water (45:55) gave TAC-1 (72 mg; M.P. 174-175). Elution of the column with methanol:water (60:40) gave TAC-2 (82mg; M.P. 182-184). Methanol wash of the column on concentration gave 1.70g of a material. This on rechromatography on RP (C-18) silica gel and elution with acetonitrile:water (50:50) gave TAC-3 (49mg) and TAC-4 (24mg).Elution with acetonitrile:water (60:40) gave TAC-5 (80mg). References: 1. Tao, C., Taylor, CM., 2011. Tarenna. In: Flora of China 19, 339-345 (http://www.efloras.org/florataxon.aspx?flora-id=2&taxonid=132320) [accessed on 6th April, 2012]. 2. Chopra R.N., Nayar S.L., Chopra L.C., (1956) Glossary of Indian Medicinal Plants. Council of Scientific and Industrial Research, New Delhi, pp. 3. Joshi,B S; Rane D F, Tetrahedron Letters (1967) ,8 (46), 4579. 4. N Subramanian, SS and Nair AGR, Phytochemistry (1971), 10(9), 2125. 5. Takeda, Yoshio; Nishimura, Hiroshi; Inouye, Pharmaceutical Bulletin (1976), 24(6), 1216-18 Hiroyuki. Chemical & 6. Ueda, S.; Iwahashi, Y. From Naturwissenschaften (1991), 78(4), 171 7. Yang, Xian-Wen; Ma, Yan-Ling; He, Hong-Ping; Wang, Yue-Hu; Di, YingTong; Zhou, Hua; Li, Ling; Hao, Xiao-Jiang. Journal of Natural Products (2006), 69(6), 971-974 8. Yang, Xian-Wen; Wang, Jun-Song; Wang, Yue-Hu; Xiao, Hai-Tao; Hu, Xu-Jia; Mu, Shu-Zhen; Ma, Yan-Lin; Lin, Hua; He, Hong-Ping; Li, Ling; et al. Planta Medica (2007), 73(5), 496-498. 9. Yang, Xian-Wen; He, Hong-Ping; Du, Zhi-Zhi; Liu, Hai-Yang; Di, Ying-Tong; Ma, Yan-Lin; Wang, Fang; Lin, Hua; Zuo, Yi-Qing; Li, Ling; et al ., Chemistry & Biodiversity (2009), 6(4), 540-550. 10. Ueda, Shinichi; Kobayashi, Koji; Muramatsu, Takeharu; Inouye, Hiroyuki. Planta Medica (1981), 41(2), 186-91 11. Zhao, Zhimin; Matsunami, Katsuyoshi; Otsuka, Hideaki; Shinzato, Takakazu; Takeda, Yoshio. Chemical & Pharmaceutical Bulletin (2008), 56(8), 1153-1158 12. Zhao, Zhimin; Matsunami, Katsuyoshi; Otsuka, Hideaki; Shinzato, Takakazu; Takeda, Yoshio. Chemical & Pharmaceutical Bulletin (2011), 59(7), 902-905. 13. Salmoun, M.; Braekman, J. C.; Ranarivelo, Y.; Rasamoelisendra, R.; Ralambomanana, D.; Dewelle, J.; Darro, F.; Kiss, R.From Natural Product Research (2007), 21(2), 111-120