Practice Exam 1 Key
advertisement

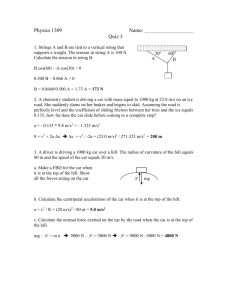
Physics 1310 Key Practice Exam 1 1. A chemistry student heats a 200 gm aluminum rod to 200ºC. The rod is 10.00 cm long. She then drops the rod into an insulated container holding 500 gm of water at 10ºC. When the water and the aluminum rod come to equilibrium, by how much has the length of the rod changed? Note: The coefficient of linear expansion for aluminum equals 24x10-6 ºC –1 and the specific heat of aluminum equals 900 J/kg ºC. The specific heat of water equals 4186 J/kg ºC. First we need to get the final T: 0.200 kg x 900 J/kg ºC x (200ºC – T) = 0.500 kg x 4186 J/kg ºC (T – 10ºC) T = 25ºC Now, calculate L: L = 24x10-6 ºC –1x 10.00 cm x (25ºC – 200ºC) = - 0.042 cm 2. A physics professor puts 2 moles of nitrogen gas into a container at 100 K. The absolute pressure inside the container equals 1.00 atmospheres. He then heats the gas by an isochoric process until the temperature of the gas equals 300K. What is the absolute pressure in the container now? Ideal Gas: pV/T = constant. Here V = constant. So p/T = constant p = 300 K/100 K x 1.00 atm = 3.00 atm 3. A geoscience student puts 2 moles of air into a container at 300 K. She wants to heat the gas to a temperature so that the rms speed of CO2 equals 1000 m/s. To what temperature should she heat the gas? Note: the molar mass of CO2 equals 44 gm/mol. v = sqrt( 3 kT / m) = sqrt (3RT/M) T = M x v2 / 3R = 44x10-3 kg / mol x 1.000x106 m2/s2 / 24.9 J/mol-K = 1767 K = 1.8 x103 K 4. A biology student places 100 g of ice at -30C into a very well insulated container that holds 600 g of water at 80C. Calculate the temperature of this system when it comes to equilibrium. Note: The specific heat of ice equals 2090 J/kg ºC, the specific heat of water equals 4186 J/kg ºC, and latent heat of fusion of water equals 333 J/g. 100 g * 2.090 J/g ºC * 30ºC + 100 g *333 J/g + 100 g * 4.186 J/g ºC * T = 600 g * 4.186 J/g ºC * (80ºC – T) T = 55 ºC 5. A field biologist is using an insulated container with dimensions equal to 20 cm x 20 cm x 20 cm. (The total surface area of the exterior container walls equals 0.240 m2.) The thickness of the container walls equals 3.00 cm. The thermal conductivity of the container material equals 0.040 W/m-K. The temperature outside the container equals 20°C. She places 500 g of ice at 0ºC inside the container. How long will it take for all the ice in the container to melt? Note: The specific heat of ice equals 2090 J/kg ºC, the specific heat of water equals 4186 J/kg ºC, and latent heat of fusion of water equals 333 J/g. H = 0.040 W/m-K * 0.240 m2 * 20ºC / 0.03 m = 6.4 W 6.4 W * time = 500 g * 333 J/g = 1.67 x105 J time = 7.2 hours 6. A tungsten filament with surface area equal top 8.0x10-5 m2 and emissivity equal to 0.25 has temperature equal to 3000K and is in an environment with temperature equal to 300K. How much power does this filament radiate? Note: The Stefan-Boltzmann constant equals 5.67x10-8 W/m2 K4. P = 0.25 * 5.67x10-8 W/m2 K4 * 8.0x10-5 m2 * [(3000 K)4 - (300 K)4] = 92 W 7. Consider 2.00 moles of an ideal gas at atmospheric pressure and 300 K. The specific heat at constant volume of this gas equals 12.5 J/mol ºC. As 200 J of heat enters the gas, it undergoes an isobaric expansion. Calculate the change in the internal energy of the gas during this process. Cp = Cv + R = 20.9 J/mol ºC Q = 200 J = 2.00 mol * 20.9 J/mol ºC * T T = 4.78 ºC U = 2.00 mol * 12.5 J/mol ºC * 4.78 ºC = 120 J 8. Consider 2.00 moles of an ideal gas at atmospheric pressure and 300 K. The specific heat at constant volume of this gas equals 12.5 J/mol ºC. The gas goes through a cycle, returning to the initial conditions. During this cycle, 500 Joules of heat enters the gas from a reservoir at 600K and 400 Joules of heat leaves the gas and flows into a reservoir at 300K. Calculate the amount of work done by the gas in this cycle. W = QH – QC = 100 J 9. A heat engine takes in 50 kJ of heat from the hot reservoir and transfers 30 kJ of heat into the cold reservoir. Calculate the efficiency of this engine. W = 20 kJ eff = 20 kJ / 50 kJ = 40% 10. A heat engine operates between a hot reservoir at 100ºC and a cold reservoir at 0ºC. Calculate the maximum possible theoretical efficiency of this engine. effcarnot = 100 K / 373 K = 27% 11. A 200 g ice cube with temperature equal to 0ºC is placed in a large tank of water with temperature equal to 27ºC. Calculate the change in entropy of the water in the tank as this system comes to equilibrium. S = Q/T = -(200 g * 333 J/g+200g*4.186J/gºC*27ºC) / 300K = - 297 J/K 12. In the diagram, q1 = 5.0 C, q2 = -5.0 C, q3 = 5.0 C, and d = 30 cm. Calculate the net force that q2 and q3 exert on q1. q2 F = 9x109 * (25x10-12 /9 x10-2 - 25x10-12 /36 x10-2) N = 1.88 N (to the right) 13. In the diagram, q1 = 5.0 C, q2 = -5.0 C, and d = 30 cm. Calculate the electric field at point P. E = 9x109*(-5x10-6 /9 x10-2-5x10-6 /9 x10-2)N/C = 1.0x106 N/C (to the left) d d + q1 + q3 d d q2 + q1 P 14. A small sphere with mass equal to 50 g has a charge equal to 6.00x10-6C. The sphere is suspended by a massless string and placed in a uniform electric E field. The equilibrium position of the sphere is shown in the figure to the right. Calculate the magnitude of the electric field. mg / cos(36.9º) = qE / sin(36.9º) E = (mg /q) tan(36.9º) = 61,000 N/C + 36.9 E 15. In the diagram to the right, the magnitude of the electric field equals 12,000 N/C. Calculate the change in electric potential as a positively charged particle with charge equal to 5.0 C moves from point A to point B. V = - E y = - 12,000 N/C * 0.04 m = - 480 volts 8 B 6 y(cm) E 4 E E E 2 A 0 0 2 4 6 8 10 12 14 x(cm) 16. In the field described in problem 15, a proton (m = 1.67x10-27 kg and q = e =1.60x10-19 C) is released from rest at point A. What is the speed of this particle when it hits one of the plates? KE = ½ mv2 = -q V v = sqrt (-2 q V / m) = sqrt ( 2 * 1.60x10-19 C * 720 V / 1.67x10-27 kg) = 3.7x105 m/s 17. A parallel plate capacitor is composed of two plates; the surface area of one side of each plate equals 0.50 m2. These plates are separated by 1.0 micrometers. The electric potential difference between these two plates equals 2000 volts. The space between the plates is filled with a substance with dielectric constant equal to 100. Calculate the magnitude of the charge stored on each plate of this capacitor. C = 100 * 8.85x10-12 C2/N-m2 * 0.50 m2 / 1.0x10-6 m = 443 F Q = 443 F * 2000 V = 0.885 C 18. The resistivity of tungsten equals 5.6x10-8 -m. Calculate the electrical resistance of a tungsten filament with diameter equal to 0.020 mm and length equal to 20 cm. the power dissipated in the light bulb. R + R = 5.6x10-8 -m * 0.20 m / [3.14 * (1.0x10-5 m)2] = 36 19. In the circuit, the battery emf equals 9.0 volts, the volatage drop across the series resistor equals 3.0 volts and the resistance of the series resistor, Rs, equals 10.0 . Calculate s I = 3.0 volt /10.0 = 0.30 amp - V = 6.0 volt P = 1.8 Watt 20. For the circuit, = 18.4 volts, Rd = 10.0 K , C =10.0 F. The capacitor is initially fully charged and the switch moves from c to d at time t = 0.00. Calculate the current flowing through Rd at t = 0.10 seconds. RC = 0.100 sec VC = 18.4 volt * e-1 = 6.77 volt I = 6.77 volt / 10 k = 0.677 mA Rc c d + sw itc h + - Rd - C