Genetics of tuberculosis in Irish dairy cows
advertisement

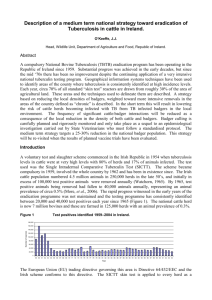
Genetics of tuberculosis in Irish dairy cows M.L. Bermingham1, S. J. More2, M. Good 3, A.R. Cromie4, and D.P. Berry1 1 Moorepark Production Research Centre, Fermoy, Co. Cork, Ireland Centre for Veterinary Epidemiology and Risk Analysis, University College Dublin, Belfield, Dublin 4, Ireland 3 Department of Agriculture and Food, Kildare St, Dublin 2, Ireland 4 The Irish Cattle Breeding Federation, Bandon, Co. Cork, Ireland 2 Tuberculosis has been an on going problem for the Irish cattle industry for many years. Prior to the introduction of the ‘test and slaughter’ policy in 1954 bovine tuberculosis was wide spread, with an animal infection level of 3.0% (140,000 reactors per annum). Progress was rapid during the initial stages of the programme, when animal infection level was reduced to 0.4% (23, 000 reactors) by 1965 when Ireland received ‘official tuberculosis free status’. However progress has stalled at this point; in 2006 animal infection level was 0.4% (26,000 reactors). Current control measures, which include annual testing of every bovine each year, have effectively minimised cattle-to-cattle transmission of infection. However, there are three main factors constraining the eradication of tuberculosis in Ireland including: the continuing expansion of the national herd, the frequency of movement during the production cycle, and the presence of a wildlife reservoir, the Eurasian badger Meles meles. Furthermore, the current programme represents a very substantial cost to the industry and government (approximately €80 million per annum). Selection for increased resistance to tuberculosis susceptibility provides an alternative strategy that could enhance existing control programmes. However, this strategy requires additive genetic variation for resistance to tuberculosis. There is some evidence at the genus level that Bos indicus is more reistant to tuberculosis than B. taurus. Furthermore, there is evidence that certain family lines are more resistant than others in; a heritability ranging from 0.06-0.08 has been estimated in cattle in Latvia. Similarly, a heritability estimate of 0.06 has been published for an analogous disease, paratuberculosis (Johne’s) disease in Dutch dairy cattle. Nevertheless, there is a lack of information on genetic parameters for tuberculosis resistance in cattle, which is largely a result of the lack of suitable data. Ireland is uniquely placed to address this issue, given the availability of three national databases (including the: AHCS [Animal Health Computer System], CMMS [Cattle Movement Monitoring System] and ICBF [Irish Cattle Breeding Federation database]) with linkable animal and herd level records of animal breeding and disease control. Furthermore, the sound epidemiological and immunological knowledge generated in Ireland and abroad were an invaluable resource in the development of a trait definition, data editing criteria and for the selection of herd and animal level risk factors to be included in analyses. The objective of this study was to estimate the genetic variation in tuberculosis resistance in Irish dairy cows. A total of 15,321 animals (including 1,268 tuberculosis positive animals) from 724 herd test dates during the period of 1st November 2002 and 31st October 2005 were available for inclusion in the analysis. All animals were Holstein-Friesian and had an identified sire. Tuberculosis positive animals were defined as animals with a skin change where the Mycobacterium bovis purified protein derivative response was 4 mm greater than that of the M. avium purified protein derivative response. Animal linear, animal threshold and maternal effects linear models were used to estimate the additive genetic and residual variance for tuberculosis susceptibility. Fixed effects in the models included, herd episode, month of test, age at test, year of calving, month of calving, proportion of Holstein genes, heterosis and recombination loss. Heritability of tuberculosis susceptibility was 0.0317 (SE=0.0106). The animal threshold model estimated an almost four fold larger heritability (h2=0.1189 (0.0281). Threshold models have been shown to be theoretically better, and are more accepted for all or nothing (reactor/non reactors) trait heritability estimation, indicating the higher heritability estimate should contribute to greater genetic improvement. The heritability estimate from the maternal effects model was 0.0285 (SE=0.0110), which was less than the estimate of the animal linear model, indicating that maternal inheritance dose not influence tuberculosis resistance in cows. The low heritability estimates observed are characteristic for disease traits, and are similar the earlier estimates of 0.06-0.08 and 0.06 for tuberculosis and paratuberculosis resistance respectively. This study has demonstrated that exploitable genetic variation for tuberculosis susceptibility exists among Irish dairy cows, and that selecting for increased resistance should be possible. The results from this study will be used to generate of breeding values for the industry, allowing breeders to identify Irish and foreign animals that are genetically resistant to infection with tuberculosis. Furthermore, while selecting for increased resistance to tuberculosis, breeders maybe indirectly selecting for general health, i.e. heightening resistance against a variety of pathogens that infect cattle in a similar manner. The breeding values estimated from this study will also be used in a collaborative project in Trinity collage Dublin, to identify the major genes associated with tuberculosis resistance. This additional knowledge will provide for more (accurate) information on the genetic merit of animals, which will be used to enhance selection programmes in the future. Increasing the resistance of our national herd to tuberculosis would prove synergistically beneficial with existing eradication efforts, through reduced animal/herd incidence, resulting in reduced animal/environmental bacterial loads, ultimately reducing cattlecattle, cattle-environment, cattle-wildlife, and environment-wildlife transmission of the disease, a major step toward the eradication of tuberculosis, and attainment of the much coveted official bovine tuberculosis free status in Ireland. The authors wish to acknowledge project funding from the Eradication of Animal Disease Board (ERAD).