Thermodynamics Writing Sample
advertisement

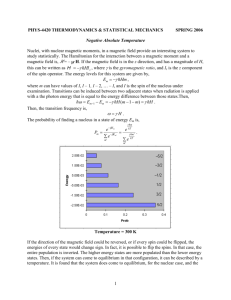
The Theoretical Description of an Adiabatic Demagnetization of a Paramagnetic Salt Nick Good, Michael Steury, Steven Cress Abstract: The complete theoretical description of the adiabatic demagnetization process was found using thermodynamical principals. This explanation was Key Words: Paramagnetic- substances with unpaired electrons. Adiabatic- a closed system where there is no change in energy. Isothermal- a closed system where there is no change in temperature. Introduction: Adiabatic demagnetization is the lowering of a magnetic field applied to a paramagnetic material that has been thermally isolated from the environment. A combination of increasing and lowering magnetic fields is used to lower the overall temperature of the paramagnetic material. This combination induces a drop in the temperature of the paramagnetic material, allowing us to obtain very low temperatures (below 1 K). This supercooling of materials is being developed into commercial uses for energy efficient refrigeration techniques and also in the study of cryogenic preservation of materials. Work for using a demagnetization process for the cooling of materials began back in 1881by Emil Warburg when he observed the effects of cooling of iron down to temperatures around 1 K. Major advances in the technique were later proposed in the 1920's via adiabatic demagnetization through the independent work of Debye (1926) and Giauque (1927). This cooling technique was first experimentally performed by scientists William F. Giauque and Dr. D.P. MacDougall in 1933 for cryogenic purposes reaching temperatures of .25 K. In 1997 a near room temperature magnetic refrigerator was demonstrated using the demagnetization technique by Prof. Karl A. Gschneidner, Jr. at the Iowa State University in Ames Laboratory. This experiment caught the attention of commercial companies along with the scientific eye. This style of refrigeration when perfected can be applied to any process involving heating, cooling, or power generation creating a method that reduces the environmental footprint of appliances that we use today.1 Methods: Like all thermodynamic assessments, the foundation must be built around the four potentials. We began by defining E, the energy of the system. This was obtained by first recognizing that is in the same format as E = U Taking the derivative of the latter equation and substituting dU we obtained the first of the four thermodynamic potentials. Then using Legendre transformations we were able to obtain the other three: Using the four thermodynamic potentials we acquired the subsequent Maxwell relations: By the potentials, the analogous relations are P and V with and M respectively. Analysis: Using the potential for energy, the change in entropy can be defined by Then using Pfaff's differential form, an equation of E (T,H) can be obtained as Combining these two equations, we have Due to constant entropy dS = 0, and the definition of specific heat being , the equation results in (1) This is the first equation of the temperature change, with a change in the magnetic field intensity at constant entropy. Our next step is to define the partial derivative remaining on the right hand side of the equation. Going back to our potential for energy, we find that Using a Maxwell Relation, we are able to substitute to obtain With the definition of the magnetization of the material M and the Curie-Weiss theory assuming becomes This simplifies to yield This gets substituted back into equation (1) and simplified to obtain (2) Using contour integration along the lines of (T, ) we found the specific heat to be (3) Putting this into equation (2) we find (4) to be our complete theoretical adiabatic description in closed algebraic form. A graphical depiction of the expression is This shows us temperature vs. magnetic field intensity. Discussion and Conclusions: The complete theoretical adiabatic expression was found to be (4)for the change in temperature with respect to change in magnetic field intensity at constant entropy. This is an explanation for the expected temperature drop for a known change in magnetic field intensity within a paramagnetic sample. From this explanation, we can make concrete, qualitative statements about this change. For the temperature to be decreasing, must be greater than or equal to one. This is due to the fact that it is squared in the denominator. If it is less than one, as approaches zero, the total value will grow infinitely large. Graphically, we are able to observe that as the magnetic field decreases the temperature approaches absolute zero. However, because of realistic limitations, actually reaching absolute zero, is impossible. In our calculations, we assumed that integrable expression. If expression. Because was approximately zero. This allowed us to obtain an were to be a non-zero value, this would have affected our final is subtracted in the denominator in the Curie-Weiss theory, as it increases, the term X would increase as well. This would result in an increase in the magnetic field intensity. Likewise, in our final expression, if the numerical constant b were to be very large, this would cause the change in temperature to be small. An interesting aspect about this final algebraic form is that the equation is completely independent of both pressure and volume. This is because volume change is so small that it becomes negligible. Because the magnetic field can only be so large, there is a limitation on the step size of the adiabatic and isothermal processes. Consequently this limits the temperature digression towards zero, making the ability to reach absolute zero physically and theoretically unattainable. References: 1. http://imagine.gsfc.nasa.gov/docs/teachers/lessons/xray_spectra/background-adr.html