phys-4420 thermodynamics & statistical mechanics spring 2003
advertisement

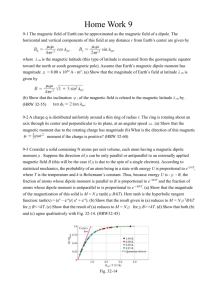
PHYS-4420 THERMODYNAMICS & STATISTICAL MECHANICS Quiz 2 SPRING 2003 Friday, April 18, 2003 NAME: ____________________________________________ To receive credit for a problem, you must show your work, or explain how you arrived at your answer. 1. (15%) The Helmholtz Free Energy is defined as F = E – TS, where E is internal energy, T is F , where temperature, and S is entropy. For a system where dW = pdV, show that S T V , N N is the number of particles in the system. 2. (40%) One mole of an ideal monatomic gas traverses the idealized Diesel cycle shown in the figure. Process 12 is adiabatic, and process 23 takes place at constant pressure. Process 34 is also adiabatic, and process 41 takes place at constant volume. (Hint: for one mole of an ideal monatomic gas, the internal energy is E 32 RT .) The temperatures of the gas at points 1, 2, 3, and 4 are: T1 = 300 K, T2 = 1600 K, T3 = 1800 K, 1 T4 = 365 K a) (10%) Find V1/V2, the ratio of the volume of the gas at point 1 to the volume at point 2. (This is the compression ratio for the Diesel cycle.) V1/V2 = _____________ b) (10%) Find the magnitude of the heat added to the gas in the process 23. Express the answer in terms of the gas constant R. Q1 = _____________ c) (5%) Find the magnitude of the work done by the gas in the process 23. Express the answer in terms of the gas constant R. W23 = _____________ d) (5%) Find the magnitude of the heat removed from gas in the process 41. Express the answer in terms of the gas constant R. Q2 = _____________ e) (5%) How much work is done by the gas in one complete cycle? Express the answer in terms of the gas constant R. W = _____________ 2 f) (5%) Find the efficiency of a heat engine operating with this cycle. = _____________ 3. (15%) A thin walled vessel of volume V contains N particles of gas which slowly leak out through a small hole of area A. No particles enter the volume through the hole. (You can imagine the vessel is in outer space.) Find the time required for for the number of particles in the vessel to decrease to N/2. Assume that the temperature within the vessel remains constant. Express your answer in terms of A, V, and v . t1/2 = ____________________ 3 4. (30%) Consider a solid consisting of N atoms, each of which has a magnetic dipole moment. When a magnetic field is applied to the solid, each dipole either points parallel to the magnetic field or antiparallel to the magnetic field (nothing else is allowed). The interaction energy of a dipole that is parallel to the field is – H, and one that is antiparallel to the field is H, where is the magnitude of a dipole moment, and H is the magnetic field strength. The system is contact with a heat reservoir which is at temperature of T. a.) (5%) What is the partition function for a single dipole? (Note that it has two energy levels.) = ____________________ b.) (5%) What is the partition function for the entire system of N dipoles? (Note that the N dipoles are distinguishable because they are in particular positions.) You can give your answer in terms of from part (a). Z = ____________________ c.) (5%) What is the average energy of the N-dipole system at temperature T? E = __________________ d) (5%)What is the energy of this N-dipole system in the limit of T0? There is no need to do a calculation in this case. Simply present a well-reasoned argument if you prefer. ET 0 = __________________ 4 e) (5%) If the magnetic field is turned off, while the solid is at a temperature close to T = 0, the energy of the spin system will change. Based on your previous answers, calculate the change of the energy. Be careful, signs are important. A positive answer means the energy increases, and a negative answer means the energy decreases. E = __________________ f) (5%) As stated earlier, the spin system is in contact with a heat reservoir. If the heat reservoir is finite, its temperature can change if heat is added or removed. Suppose it is finite. Based on your work so far, what will happen to the temperature of the reservoir when the magnetic field is turned off. (Circle the correct answer.) THE TEMPEATURE WILL INCREASE THE TEMPERATURE WILL DECREASE THE TEMPERATURE WILL REMAIN THE SAME 5