Plasma Total Homocysteine Concentrations Are Unrelated to Insulin
advertisement

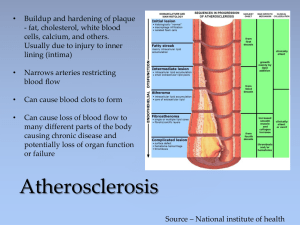
Plasma Total Homocysteine Concentrations Are Unrelated to Insulin Sensitivity and Components of the Metabolic Syndrome in Healthy Men1 I. F. Godsland, J. R. Rosankiewicz, A. J. Proudler and D. G. Johnston Endocrinology and Metabolic Medicine, Imperial College School of Medicine, London, United Kingdom W2 1PG Address all correspondence and requests for reprints to: Ian F. Godsland, Ph.D., Endocrinology and Metabolic Medicine, Imperial College School of Medicine, Norfolk Place, London, United Kingdom W2 1PG. E-mail: i.godsland@ic.ac.uk. Plasma homocysteine levels are lowered by insulin and can be elevated in insulinresistant states. However, it is uncertain whether homocysteine and insulin resistance or components of the metabolic (insulin resistance) syndrome are related in healthy individuals. Total homocysteine concentrations were measured by gas chromatographymass spectrometry in samples from 100 male participants in the second follow-up cohort of the Heart Disease and Diabetes Risk Indicators in a Screened Cohort Study. Members of this cohort have each undergone an iv glucose tolerance test with measurement of insulin sensitivity by minimal model analysis. Age ranged from 31–62 yr (mean, 46.8), body mass index from 20.6–36.5 kg/m2 (mean, 26.3), insulin sensitivity from 0.0–9.6 min/mU·L (mean, 2.32), and homocysteine concentrations from 7.5–30.6 µmol/L (mean, 12.2). In univariate correlation, homocysteine concentrations were unrelated to insulin sensitivity or to components of the metabolic syndrome, including fasting serum triglycerides, high density lipoprotein cholesterol, high density lipoprotein subfraction 2 cholesterol, blood pressure, uric acid, systolic blood pressure, or body mass index. These measures were, nevertheless, highly intercorrelated. These findings strengthen the possibility that in healthy humans, homocysteine metabolism is not substantially affected by insulin action. IV. Determinants of Plasma Homocysteine Top A. Physiological Abstract Several environmental factors have been found to play a I. Introduction role in determining the presence or absence of HH(e) (35 II. Methionine-Homocysteine... 36 ). Lussier-Cacan et al. (36 ) studied a large number of III. Nomenclature and... healthy men and women, excluding individuals with IV. Determinants of Plasma... major and common disorders. They determined that V. Homocysteine and Diabetes... gender was a major determinant of fasting plasma H(e) VI. Hyperhomocysteinemia and... concentration and that women had a 21% lower VII. Hyperhomocysteinemia in... concentration than men. The gender difference in H(e) VIII. Possible Mechanisms Of... concentrations between men and women persist in IX. Management of... elderly persons, although postmenopausal women have X. Conclusion higher concentrations than premenopausal women. References Plasma H(e) concentrations increase with age and remain an independent risk factor for vascular disease in the elderly (37 ). The marginal folate and other vitamin deficiencies known to be common in the elderly are likely to be contributing factors to HH(e) (38 39 ). There are significant negative correlations between plasma H(e) and serum folate and vitamin B12 concentrations. Plasma H(e) was also highest in individuals in the lowest quartile of serum pyridoxal-5'-phosphate, although this active metabolite of vitamin B6 is more important in determining postmethionine load plasma H(e) than fasting H(e) (36 ). Positive correlations have also been found between plasma H(e) and uric acid and creatinine concentrations that may be related to the links between H(e) metabolism with those of creatinine and uric acid (35 ). Plasma albumin concentration also correlates with plasma H(e) and may reflect an increase in protein-bound H(e). The exact significance of protein binding of H(e) with respect to cardiovascular disease is unknown. In the Hordaland H(e) study, elevated plasma H(e) was associated with male gender, increasing age, smoking, hypertension, elevated cholesterol, and lack of exercise (40 ). In a multivariate analysis, Malinow et al. (41 ) demonstrated that systolic blood pressure, plasma uric acid, and hematocrit were predictors of concentrations of plasma H(e) in men who did not have a history of atherosclerotic disease. Hyperhomocysteinemia Andrea Cortese Hassett Ph.D., Chief Science Officer, ITxM Diagnostics INTRODUCTION Homocysteine is a naturally occurring, sulfur containing amino acid formed during the metabolism of methionine, an essential amino acid derived from the diet. The interconversion of methionine and homocysteine depends on the availability of the methyl donor 5-methyltetrahydrofolate, cofactors vitamin B12 and folate, and the enzyme activity of methionine synthase. Elevated intracellular homocysteine concentrations with corresponding increases in blood levels can result from augmented production or reduced metabolism. Although severe hyperhomocysteinemia is rare, mild hyperhomocysteinemia occurs in approximately 5 to 7 percent of the general population.1,2 Patients with mild hyperhomocysteinemia are asymptomatic until the third or fourth decade of life when premature coronary artery disease may develop, as well as recurrent arterial and venous thrombosis. MEASUREMENT SPECIMEN REQUIREMENTS Plasma homocysteine is measured on a morning specimen collected in an EDTA (lavender top) tube after an overnight fast. Because homocysteine is continuously released by blood cells, the specimen must be centrifuged and the plasma separated immediately to avoid falsely elevated values. Alternatively, the specimen can be placed on wet ice until it can be centrifuged. Specimens that are not sent to the lab the same day must be spun down and the plasma frozen until testing is performed. METHODS Chromatography (HPLC and gas) and enzyme immunoassay are the two main analytical methods used to measure homocysteine. The latter method is simple, rapid, and has good to excellent performance data, making it suitable for routine lab analysis. Among the commercially available assays, the fluorescence polarization immunoassay is used extensively. 3,4 A methionine load challenge (100 mg/kg body weight oral dose of methionine) can be given to individuals with suspected hyperhomocysteinemia who have normal homocysteine concentrations on fasting specimens. This procedure requires measurement of plasma homocysteine concentration before the methionine challenge and between four and eight hours afterward.5 The methionine challenge test cannot adequately assess thermolabile variants of the methyltetrahydrofolate reductase (MTHFR) protein and should be utilized when assessing enzymes of the transulfuration pathway (Cystathionine b-Synthase). RESULTS Using any of the standard analytical methods, values between 5 and 15 mmol/L are generally considered normal in the fasting state, albeit not optimal (<10 mmol/L). 6,7 Kang and coworkers have classified hyperhomocysteinemia as moderate (15 to 30 mmol/L), intermediate (>30 to 100 mmol/L) and severe (>100 mmol/L) on the basis of concentrations measured during fasting. 8 Levels tend to increase with age. CAUSES Elevations in plasma homocysteine are typically caused either by genetic defects in the enzymes involved in homocysteine metabolism or by nutritional deficiencies in vitamin cofactors. Homocystinuria and severe hyperhomocysteinemia are caused by rare inborn errors of metabolism (most commonly Cystathionine beta-synthase deficiency) resulting in marked elevations of plasma and urine homocysteine concentrations. Deficiencies of the B complex vitamins and folate in particular can also cause large increases in homocysteine levels (exceeding 100 mmol/L). More recently, two common polymorphisms of MTHFR (C677T and A1298C) have been shown to contribute to moderate hyperhomocysteinemia.9,10 These mutations are associated with reduced MTHFR activity and thermolability, requiring increased levels of folate intake. Homozygosity for the C677T mutation (9-17% population) and C677T/A1298C combined heterozygosity both have been associated with increased homocysteine levels and a mild prothrombotic tendency. Nutritional deficiencies in the vitamin cofactors (folate, vitamin B12 and vitamin B6) required for homocysteine metabolism may also promote hyperhomocysteinemia. It has been speculated that these types of nutritional deficiencies contribute to approximately two-thirds of all cases of hyperhomocysteinemia.11 In addition to vitamin deficiencies, several therapeutic drugs (methotrexate, theophylline, cyclosporine and most anticonvulsants) and chronic disease states (liver and renal disease, hypothyroidism and malignancies) can lead to moderate hyperhomocysteinemia. ASSOC. WITH VASCULAR DISEASE High homocysteine levels can damage blood vessels in several ways, including injury to arterial endothelial cells and promotion of smooth muscle growth, both of which result in lesions (plaques) that narrow the lumens of the affected vessels. Increased homocysteine concentrations can also disrupt normal blood clotting mechanisms, increasing the risk of thrombi formation that can lead to heart attack or stroke. A growing body of literature indicates that elevated homocysteine is a risk factor for coronary, cerebrovascular, and peripheral atherosclerotic disease, as well as arterial and venous thrombosis. A homocysteine level above 15 mmol/L is associated with a significantly higher risk compared to lower levels. Furthermore, plasma homocysteine is independent of, but interacts with, conventional coronary vascular disease (CVD) risk factors, enhancing their effect. TREATMENT Vitamin therapy and dietary modification can work together to help lower plasma homocysteine levels. Blood levels of homocysteine become elevated if the dietary folic acid intake is <250 mg per day, so dietary folic acid supplementation and maintaining an adequate intake of vitamins B 6 and B12 is also recommended. Studies are currently in place to determine whether or not normalizing homocysteine levels will improve cardiovascular morbidity and mortality. SUMMARY Homocysteine has been shown to be an independent risk factor for the development of vascular disease. Homocysteine measurements should be included in the evaluation of individuals in high-risk groups. These groups include patients with: 1) Evidence of increased urinary homocysteine 2) Premature arteriovascular disease 3) Strong family history of: a. Myocardial infarction b. Peripheral vascular disease c. Stroke d. Recurrent pulmonary embolism e. Venous thrombosis f. Renal Failure g. Cardiac or renal transplant For the regular use of homocysteine testing as a marker of CVD and the use of folic acid supplementation to prevent CVD, the medical community will have to wait for the results of the numerous ongoing outcomes studies. REFERENCES