Homework # 3: Acids and Esters
advertisement

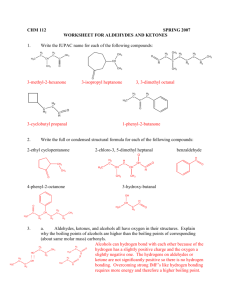
Week 4: Acids and Esters Lecture # 9, 10, 11 Concepts: Acids, ionization, boiling point.polarity, solubility, salts, neutralization Reaction Summary: Write the word equations. Self Ionization of Acids in Water Neutralization of Acid with Base Synthesis of Acids Ester Synthesis Ester Hydrolysis acidic and basic Ques. 1. Which compound in each pair will have the highest boiling point and thus be the most polar? a. b. c. d. e. heptane or nonane 1-propanol or methyl ethyl ether 1-propanol or propylamine 1-propanol or propanoic acid 1 butanol or methyl propanoate Ques. 2. Rank the following compounds in order of increasing polarity. 1 = most non-polar. CH3OH, CH3CH3, CH3NH2, CH3-OCH3, CH3COOH, CH3OOCCH3 Ques. 3. A. Rank the following compounds in order of increasing solubility in water. 1 = most insoluble. pentanoic acid, pentane, 1-pentanol, ethyl propyl ether B. Rank the following compounds in order of increasing solubility in water. 1 = most insoluble. Ethanoic acid, hexanoic acid, butanoic acid, methanoic acid Ques. 4: Show how acetic acid can hydrogen bond to make a dimer. Also show how acetic acid can hydrogen bond with water, but that methyl ethanoate is unable to hydrogen bond to each other. Ques 5. Rank the following compounds in order of increasing solubility in hexane. 1 = most insoluble. butane, butanol, butanoic acid, diethyl ether, methyl propanoate Ques. 6. Write the reaction for the synthesis of carboxylic acids. Name the reactants and the products. H2 C A. H C H3C + Oxidizing Agent C H2 O CH3 B. H2C H3C O CH H2C + Oxidizing Agent CH Ques. 7. Write the reaction for the self ionization of carboxylic acids in water. Name the reactants and the products. O H 3C A. + H2O C C H2 OH CH3 O + H 2O B. CH H3C C C H2 OH Neutralization Reaction or Salt Formation Reaction Name Word Equation Example 20. Acid Salt reaction Acid + MOH M= Na, K, Li, metal ion acid salt Ethanoic aicd + NaOH Ques. 8. Write the reaction for the neutralization of carboxylic acids in with base to form salts. Name the reactants and the products. O + KOH A. C OH H3C H3C B. H3C O CH + KOH C OH 12. Ester Synthesis Alcohol + acid ester + water Methanol + ethanoic acid Ques. 9. Write the reaction for the synthesis of ethers and esters. Name the reactants and the products. H2 C A. H3C OH H3C H2 C HO C H2 CH3 O H2 C B. + OH + C HO CH3 C H2 CH3 O C. H3C C + HO C OH H2 Ques. 10: Write the names of the following: CH CH3 Ques. 11: Synthesis of Esters Write names of reactants and products: a. O H2 C H3C OH H2 C + H3C C H2 b. + C OH H2 C O H3C C HO H2 C C H2 CH3 butanol OH c. O H2 C H3C C H2 + C C H2 OH H3C OH d. O H3C OH + H3C C C H2 OH Hydrolysis Reactions - add water to split a molecule (reverse of combination reactions) Reaction Name Word Equation Example 17. Ester Hydrolysis Ester + HOH Methyl ethanoate + HOH alcohol + acid Ques. 12: Hydrolysis of Esters: Name the reactants and products a. O H2 C H3C H2 C C H2 C O CH3 H2 C CH3 + HOH + HOH b. O H3C C O C H2 C H2 Basic Hydrolysis (NaOH) Second step: neutralize acid with NaOH c. O H3C C O C H2 + CH3 HOH Basic Hydrolysis (NaOH) Second step: neutralize acid with NaOH Ques. 13. Write the reaction for the hydrolysis of esters. Name the reactants and the products. O Acid Hydrolysis H2 C A. H3C CH3 C O CH2 C H2 O B. H3C C O CH3 + NaOH (Basic Hydrolysis) Ques. 14. Neutralize the following fatty acid with NaOH. Then explain the specifics of how the soap molecule works to clean dirt. How does it act as a “bridge” between oil and water? Use the concepts of micelle, polar and non O HO polar. Ques. 15. Write the reaction for the synthesis of ethers and esters. Name the reactants and the products. OH C O C + H3C OH could get two products C OH OH C O O + C C C OH OH aspirin H 3C b. Which structure above is salicylic acid, aspirin and which is oil of winter green? Ques. 16. Explain how a polyester is made. You do not need to write the reaction, but do need to explain how the reaction is similar but different from the other ester synthesis we have been doing.