Figure 1. CD spectra of a gel of 1 and of 1 + ba
advertisement
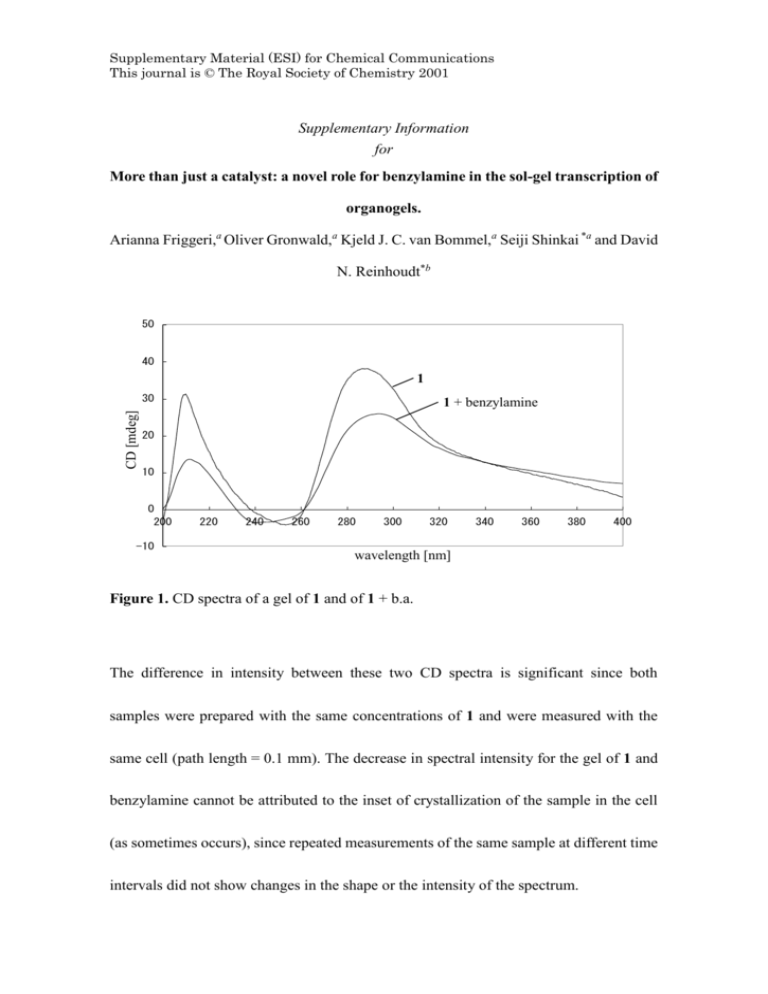
Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2001 Supplementary Information for More than just a catalyst: a novel role for benzylamine in the sol-gel transcription of organogels. Arianna Friggeri,a Oliver Gronwald,a Kjeld J. C. van Bommel,a Seiji Shinkai *a and David N. Reinhoudt*b 50 40 1 CD [mdeg] 30 1 + benzylamine 20 10 0 200 -10 220 240 260 280 300 320 340 360 380 400 wavelength [nm] Figure 1. CD spectra of a gel of 1 and of 1 + b.a. The difference in intensity between these two CD spectra is significant since both samples were prepared with the same concentrations of 1 and were measured with the same cell (path length = 0.1 mm). The decrease in spectral intensity for the gel of 1 and benzylamine cannot be attributed to the inset of crystallization of the sample in the cell (as sometimes occurs), since repeated measurements of the same sample at different time intervals did not show changes in the shape or the intensity of the spectrum. Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2001 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2001 4000 3500 3000 2500 2000 2526 A (a.u.) A (a.u.) 4500 4500 4000 3500 4500 4000 3500 3000 2500 2000 3000 2500 2000 d A (a.u.) 3254 3415 c A (a.u.) Absorbance (a.u.) 3377 b 3532 3337 a 4000 3500 3000 2500 2000 4500 -1 wavelength [cm ] 3482 Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2001 Figure 2. FT-IR spectra of amorphous compound 1 (a), amorphous compound 1 + b.a. (b), xerogel of 1 (c) and xerogel of 1 + b.a. (d). FT-IR spectra (KBr pellet) of amorphous compound 1 and of amorphous compound 1 + b.a. were also measured (Figures 2a and 2b, respectively). Only the H-bonding region of the FT-IR spectra is shown since it provides the most important information about the intermolecular arrangement. In the spectrum of amorphous compound 1 (Figure 2a) both free (sharp peak at 3532 cm-1) and H-bonded (broad peak at 3337 cm-1) OH groups belonging to the glucose moiety are present. However, in the xerogel of 1 (Figure 2c), the more ordered packing of the molecules in the gel with respect to the amorphous solid leads to only one, broad peak at around 3415 cm-1, corresponding to H-bonded OH groups.1 The spectrum of amorphous compound 1 + b.a. (Figure 2b) is similar to that of amorphous compound 1 alone (Figures 2a), with the additional vibrations for the free and H-bonded NH group of the benzylamine (sharp peak at 3377 cm-1 and broad peak at 2526 cm-1, respectively). Supplementary Material (ESI) for Chemical Communications This journal is © The Royal Society of Chemistry 2001 b.a. 343 1 : 1.0 333 1 : 1.0 323 1 : 1.8 313 1 : 2.9 303 1 : 2.9 298 1 : 1.5 / ppm 9.0 8.5 8.0 7.5 1 : b.a. T/K 1 7.0 Figure 3. Temperature dependent 1H NMR spectra showing the aromatic protons region for 1 + b.a. (gel at 298 K, sol at 343 K); the molecular ratio of 1 : b.a. is shown on the right of each spectrum, as determined from the integrals of the corresponding NMR peaks. 1 K. Inoue, Y. Ono, Y. Kanekiyo, T. Ishi-i, K. Yoshihara and S. Shinkai, J. Org. Chem., 1999, 64, 2933; K. Yoza, N. Amanokura, Y. Ono, T. Akao, H. Shinmori, M. Takeuchi, S. Shinkai and D. N. Reinhoudt, Chem. Eur. J., 1999, 5, 2722; N. Amanokura, K. Yoza, , H. Shinmori, S. Shinkai and D. N. Reinhoudt, J. Chem. Soc., Perkin Trans. 2, 1998, 2585.




